Sodium hydrosulfite
-
Post Date:
Apr 19,2025 -
Expiry Date:
Oct 16,2025 -
Detailed Description:
Cas No. :7775-14-6
Alias: sodium dithionite
Molecular formula: Na2S2O4
English name: Sodium Hydrosulphite, Sodium dithionite Appearance: white crystalline powder
Relative density (water=1): 1.02
UN No.: 1384
Chemical stability: according to the recommended disposal and storage method, it is stable at normal temperature in a closed container.
Possibility of dangerous reaction: it can burn when heated or exposed to open flame. Exposed to the air will be oxidized and deteriorated. In contact with water, acids or organic substances and oxidants, it can release a lot of heat and cause severe combustion.
Conditions to avoid: self-heating can occur.
Incompatible substances and materials: strong oxidants, etc.
Hazardous decomposition products: combustion products include sulfur oxides (SOx). Use: This product is used as a dyeing aid for cotton fabrics and a bleaching agent for cotton and wool fabrics. It is also used in medicine, mineral processing, copper plate printing, synthesis of thiourea and other sulfides. Paper industry is also used as bleach, and food-grade products are used as bleach, preservative and antioxidant
ITEM STANDARD Hydrosulphite 74% 85% 88% 90% Water insoluble 0.3 max 0.3 max 0.1 max 0.1 max Sodium thiosulfate: Na2S2O3 1.4 max 1.5 max 1.3 max 1.0 max Sodium metabisulfite: Na2S2O5 5-7 5-7 5-7 5-7 Sodium bisulfite:NaHSO3 0.5 max 0.5 max 0.5 max 0.5 max Sodium carbonate: Na2CO3 0.6-2 max 0.6-2 max 0.6-2 max 0.6-2 max Sodium formate: HCOONa 0.5 max 0.8 max 0.5 max 0.5 max -
CAS Registry Number:
7775-14-6 -
Synonyms:
;Sodium dithionite hydrate;Sodium hydrosulphite;Sodium dithionite;disodium dithionite; -
Molecular Formula:
Na2O4S2 -
Molecular Weight:
174.1071 -
Molecular Structure:
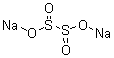
-
Hazard Symbols:
Xn:Harmful;
-
Risk Codes:
R22:;
R31:;
R7:;
-
Safety Description:
S26:;
S28A:;
S43E:;
S7/8:;
-
Company:
Shandong Weigorge Import and Export Co., Ltd. [ China ] -
Contact:
-
Tel:
-
Fax:
-
Email:
weigorge@163.com