 CN
China Suppliers > wentong >
CN
China Suppliers > wentong > year
| Category: | Inorganic chemicals |
|
|
| CAS NO: | 7757-79-1 | ||
| EC NO: | 231-818-8 | ||
| Molecular Formula: | KNO3 | ||
| Molecular Weight: | 101.1032 | ||
| Specification: | potassium nitrate | ||
| InChI: | InChI=1/K.NO3/c;2-1(3)4/q+1;-1 | ||
| Packing: | Plastic woven bag or kraft paper bag inner with plastic bag in 2 0/25/50/500/1000kg net | ||
| Product description: Potassium Nitrate is colorless and transparent rhombic crystals or white powder. The relative density is 2.109 and the melting point is at 334. It decomposes at 400 and turns into potassium nitrite releasing oxygen and then in continuous heat turns into burnt potash. It is highly soluble in water, liquid ammonia and glycerine but insoluble in anhydrous alcohol and diethyl ether. It can not deliquesce in the air. As strong oxidizer, it will burn and explode when meeting with organics releasing irritant gas. If mixed with carbon powder or sulfur, it burns with strong light. |
|||
| Uses: | Industry and Agriculture | ||
| Synonyms: | Potassium nitrate (99.999%-K) PURATREM;PotassiumnitrateACSwhitextl;Potassium ion chromatography standard solution Fluka;Nitrate Ion standard solution Fluka;Nitrate solution;Filling solution for salt bridges and double-junction electrodes;Filling solution for chloride combination electrode (10% KNO3, trace AgCl, KCl);Filling solution for fluoride electrode (17% KNO3, 5% KCl, trace NaCl, AgCl);Filling solution for cyanide or sulfide electrode (10% KNO3, trace AgCl, KCl);NO3;NITRATE SINGLE COMPONENT STANDARD;NITRATE STANDARD;NITRATE;NITRATE, CERTIFIED ANION STANDARD;NITRATE IC STANDARD;NITRATE ION; | ||
| Molecular Structure: |
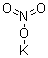 |
||