Sofosbuvir
-
- Category :
Pharmaceuticals and Biochemicals
- CAS NO : 1190307-88-0
- EC NO :
- Molecular Formula : C22H29FN3O9P
- Main Specifications : ASSAY 98.5% - 102.0%
- Synonyms : PSI 7977;Sofosbuvir(cGMP);L-Alanine, N-[[p(S),2'R]-2'-deoxy-2'-fluoro-2'-methyl-p-phenyl-5'-uridylyl]-, 1-methylethyl ester;Psi-7977;(S)-isopropyl 2-(((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate;sofosvuvir form6;sovaldi API;sofosbuvir form6;GS-7977;Sopropyl-(2S)-[[[(2R,3R,4R,5R)-5-(2,4-dioxopyrimidin-1-yl]-4-fluoro-3-hydroxy-4-methy-ltetrahydrofuran-2-yl]methoxy-phenoxy-phosphoryl]amino]propanoate;Sofasbuvir;(S)-isopropyl 2-(((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4 dihydropryimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2yl) methoxy)(phenoxy)phosphoryl)amino) propanoate;
Package: AS REQUIRED
Uses : for the treatment of chronic hepatitis C (CHC) infection as a component of a combination antiviral treatment regimen.
Molecular Structure:
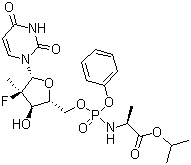
Product description:
- CAS No. 1190307-88-0
- Category: API
- Standard: In house
- Crystal Form: F1 & F6
- Certificate: DML/DMF
- Appearance: White to off-white powder
- Usage: for the treatment of chronic hepatitis C (CHC) infection as a component of a combination antiviral treatment regimen.
Main Items Specifications
Solubility Practically insoluble in water, very soluble in methanol
Water 0.5% MAX
Pentafluorophenol 20ppm MAX
SFPR13 0.1% MAX
Assay 98.5% - 102.0%
Conclusion IN-HOUSE
- Packing: as required
- Shelf life: one year
