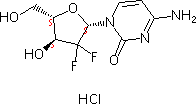
Gemcitabine Hydrobromide
-
- Category :
Pharmaceuticals and Biochemicals
- CAS NO : 122111-03-9
- EC NO :
- Molecular Formula : C9H12ClF2N3O4
- Main Specifications : 98% up, Medicine Grade
- Synonyms : 2',2'-Difluoro-2'-deoxycytidine;2,2-Difluoro-2-deoxycytidine DDFC;4-Amino-1-[3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1H-pyrimidin-2-one hydrochloride;Gemcitabine HCl;4-amino-1-(2-deoxy-2,2-difluoropentofuranosyl)pyrimidin-2(1H)-one;4-amino-1-(2-deoxy-2,2-difluoropentofuranosyl)pyrimidin-2(1H)-one hydrochloride (1:1);Gemcitabine hydrochlorid;2',2'-Difluoro-2'-deoxycytidine hydrochloride;
Package: 25kg/drum
Uses : Gemcitabine hydrochloride is a synthetic novel difluoro nucleoside drug that is anti-metabolic and antineoplastic.
Molecular Structure:
Product description:
Gemcitabine hydrochloride is a synthetic novel difluoro nucleoside drug that is anti-metabolic and antineoplastic. It is researched and developed by the Eli Lilly and Company and approved to be listed in South Africa, Sweden, the Netherlands, Australia and other countries in 1995. The United States Food and Drug Administration ( referred FDA) approved it as the first-line therapy for the clinical treatment of non-small cell lung cancer and pancreatic cancer.
Suitable for the treatment of inoperable advanced or metastatic pancreatic cancer and the treatment of locally advanced or metastatic non-small cell lung cancer, and the treatment for mid-term and advanced non-small cell lung cancer, non-small cell lung cancer, pancreatic cancer, bladder cancer, breast cancer and other solid tumors.
