Valaciclovir
-
- Category :
Inorganic chemicals
- CAS NO : 124832-26-4
- EC NO :
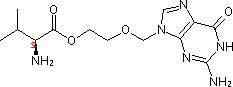
- Molecular Formula : C13H20N6O4
- Main Specifications : 99% up
- Synonyms : L-VALINE 2-[(2-AMINO-1,6-DIHYDRO-6-OXO-9H-PURIN-9YL)METHOXY]ETHYL ESTER;l-valine 2-(guanin-9-ylmethoxy)ethyl ester;VALACYCLOVIR;L-Valine ester with 9-[(2-hydroxyethoxy)methyl]guanine��L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethyl ester (9CI);
Package: 25kg/drum
Uses : Pharmaceutical Intermediate
Molecular Structure:
Product description:
Valacyclovir hydrochloride, an orally active L-valyl ester of the potent antiviral agent aciclovir, was launched in 1995 in the United Kingdom for the treatment of herpes simplex virus (HSV) infections of the skin and mucous membranes, including initial and recurrent enital herpes. As a prodrug, valaciclovir has an improved pharmacokinetic profile to aciclovir. It is rapidly absorbed after oral administration and extensively converted to aciclovir via first-pass metabolism to achieve plasma levels of aciclovir comparable to those seen with aciclovir via i.v. route. Valacyclovir is then activated selectively in virus-infected cells by viral thymidine kinase to form aciclovir triphosphate in a stepwise fashion. This active species inhibits viral DNA polymerase via irreversible binding to the active site of the enzyme. Once aciclovir is incorporated into the elongating viral DNA, it terminates replication of the viral DNA strand, an antiviral mechanism unique to aciclovir. Valacyclovir is reportedly in clinical trials for the suppression of cytomegalovirus infection and disease in renal transplant patients.
