 CN
China Suppliers > kaixianghg >
CN
China Suppliers > kaixianghg > year
| Category: | Adhesives and Sealants/Acrylic Adhesives |
|
|
| CAS NO: | 13598-36-2 | ||
| EC NO: | 237-066-7 | ||
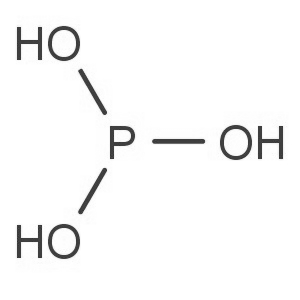
| Molecular Formula: | H3PO3 | ||
| Molecular Weight: | 81.9957 | ||
| Specification: | H3PO3:98.5% Min, Fe: 50ppm MAx, Cl: 0.01% Max, So4: 0.008% max, Po4: 0.6% max | ||
| InChI: | InChI=1/3H2O.H3P/h3*1H2;1H3/q;;;+3/p-3 | ||
| Packing: | 25kg woven bag with double PE inner or 500kg/1000kg super sack | ||
| Product description: Phosphorous acid Introduction Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. CAS NO.:13598-36-2 Appearance: white solid deliquescent Density:1.651 kg/L Melting point:74℃ Boiling point::200℃ Chemical formula: H3PO3 Molecular shape: tetrahedral Use: Industry and agriculture The most important use of phosphorous acid (phosphonic acid) is the production of phosphites (phosphonates) which are used in water treatment. Phosphorous acid is also used for preparing phosphite salts, such as potassium phosphite. These salts, as well as aqueous solutions of pure phosphorous acid, are fungicides. Phosphites have shown effectiveness in controlling a variety of plant diseases, in particular, treatment using either trunk injection or foliar containing phosphorous acid salts is indicated in response to infections by phytophthora and pythium-type plant pathogens (both within class oomycetes, known as water molds), such as dieback/root rot and downy mildew.[7] Anti-microbial products containing salts of phosphorous acid are marketed in Australia as 'Yates Anti-Rot'; and in the United States of America, for example, aluminum salts of the monoethyl ester of phosphorous acid (known generically as 'Fosetyl-Al') are sold under the trade name 'Aliette'. Phosphorous acid and its salts, unlike phosphoric acid, are somewhat toxic and should be handled carefully As a chemical reagent Phosphorous acid is used in chemical reactions as a reducing agent that is somewhat less vigorous than the related hypophosphorous acid. |
|||
| Uses: | Agricultural products (non-pesticidal) Not known or reasonably ascertainable Personal care products Water treatment products | ||
| Synonyms: | Phosphonic acid;hydrogen phosphonate;phosphorus(+3) trihydride cation trihydroxide;Orthophosphorus;Orthophosphorous acid;Ortho-Phosphorous acid; | ||
| Molecular Structure: |
 |
||