Hydrogen bromide
-
- Category :
Pharmaceuticals and Biochemicals/Immunomodulator
- CAS NO : 10035-10-6
- EC NO : 233-113-0
- Molecular Formula : HBr
- Main Specifications : 48% purity
- Synonyms : Hydrogen bromide;Hydobromic acid;Anhydrous hydrobromic acid;
Package: 25kg/bag etc
Uses : used for the production of inorganic bromides
Molecular Structure:
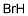
Product description:
Hydrogen bromide is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C and contains 47.6% HBr by weight, which is 8.89 mol/L. Hydrobromic acid has a pKa of −9, making it a stronger acid than hydrochloric acid, but not as strong as hydroiodic acid. Hydrobromic acid is one of the strongest mineral acids known
Uses:
Hydrobromic acid is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium. It is a useful reagent for generating organobromine compounds. Certain ethers are cleaved with HBr. It also catalyzes alkylation reactions and the extraction of certain ores. Industrially significant organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis(phenol), and bromoacetic acid.
