Heparin Injection
-
- Category :
Pharmaceuticals and Biochemicals/Blood system agents
- CAS NO : 9041-08-1
- EC NO :
- Molecular Formula : ca. (C12H16NS2Na3)20
- Main Specifications : USP30 EP6.0
- Synonyms : Ardeparin sodium;Dalteparin Sodium;Tinzaparin Sodium;Fragmin;Sodium Heparin;
Package: 1kg/aluminum foil bag 5kg/aluminum tin
Uses : used in hemodialysis to prevent blood clot formation,
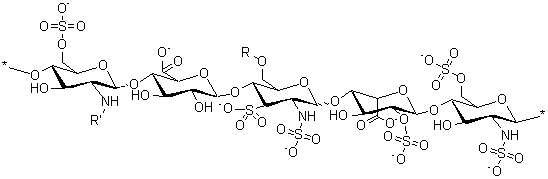
Molecular Structure:
Product description:
Heparin Injection
Pharmacological effects:The product of its plasma pharmacokinetics of anti-factor Xa activity determined. 3 hours after subcutaneous injection to reach plasma peak, then decline, but should still be monitored within 24 hours, half-life of about 3.5 hours, medication during the anti-factor IIa activity than anti-factor Xa activity, subcutaneous bioavailability close to 100%.
Pharmacokinetics:
Indications:This product is mainly used in hemodialysis to prevent blood clot formation, but also for the treatment of deep vein thrombosis.
Usage and Dosage:1. To prevent blood clot formation during hemodialysis. The beginning of each dialysis, vascular access should be infused into the arterial end of 5000 anti-Xa factor product international units, dialysis, no longer increase the dose or the doctor's advice.
2. For the treatment of deep vein thrombosis.
(1) 1 ~ 2 hours before surgery, subcutaneous injection of 2500 anti-Xa international units, post-operative day, subcutaneous injection of 2500 anti-Xa international units, after continuous medication for 5 days.
2) The abdominal subcutaneous or the doctor's advice.
Adverse reactions:Occasionally minor bleeding, thrombocytopenia, allergic reactions, injection site hematoma and mild necrosis.
Contraindications:For this product allergy, acute bacterial endocarditis, thrombocytopenia is disabled.
Note:1. The goods and non-steroidal anti-inflammatory drugs, salicylic acid medicines, oral anticoagulation drugs, drugs that affect platelet function and plasma expander,etc. should pay attention to observe the simultaneous use of drugs, because these drugs can increase the risk of bleeding sex.
2. Not intramuscular injection.
3. Used with caution in the following situations:
(1) has a history of allergies.
(2), bleeding tendency and coagulation mechanisms disabilities. Including gastric and duodenal ulcers, stroke, severe liver and kidney disease, severe high blood pressure, retinal vascular lesions.
(3) pregnant women.
4. Occurs when excessive, can be injected protamine hydrochloride or protamine sulfate and the products, a unit of protamine hydrochloride, and 1.6 anti-Xa international units of this product.
5. During treatment, attention to regular platelet count and anti-Xa factor activity.
Specifications:(1) 1ml: 2500 anti-factor Xa international units; (2) 1ml: 5000 anti-factor Xa international units.
