Heparin Sodium
-
- Category :
Pharmaceuticals and Biochemicals
- CAS NO : 9041-08-1
- EC NO :
- Molecular Formula : ca. (C12H16NS2Na3)20
- Main Specifications : Injectable grade ,EP6.0
- Synonyms : Ardeparin sodium;Dalteparin Sodium;Tinzaparin Sodium;Fragmin;Sodium Heparin;
Package: 25kg/bag
Uses : Pharmaceuticals
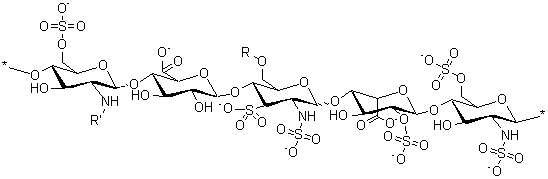
Molecular Structure:
Product description:
Heparin is a heterogeneous group of straight-chain anionic mucopolysaccharides, called glycosaminoglycans, having properties. Although others may be present, the main sugars occurring in heparin are: (1) _-L-iduronic acid 2-sulfate, (2) 2-deoxy-2- sulfamino-_-D-glucose 6-sulfate, (3) _-D-glucuronic acid, (4) 2 acetamido-2-deoxy-_-Dglucose and (5) _-L-iduronic acid. These sugars are present in decreasing amounts, usually in the order (2) > (1) > (4) > (3) > (5), and are joined by glycosidic linkages, forming polymers of varying sizes. Heparin is strongly acidic because of its content of covalently linked sulfate and carboxylic acid groups. In heparin sodium, the acidic protons of the sulfate units are partially replaced by sodium ions.
Heparin Sodium Injection, USP is a sterile solution of heparin sodium derived from porcine intestinal mucosa, standardized for anticoagulant activity, in water for injection. It is to be administered by or deep routes. The potency is determined by a biological using a USP reference standard based on units of heparin activity per milligram.
Structure of Heparin Sodium (representative subunits):
Heparin Sodium Injection, USP (porcine) is available as follows:
Each 1,000 Units/mL contains: 1,000 USP Heparin Units (porcine); 9 mg sodium chloride; Water for Injection q.s. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment (5.0-7.5).
