 CN
China Suppliers > WeiGorge >
CN
China Suppliers > WeiGorge > year
| 111Category: | Inorganic chemicals |
|
||||||||||||||||||||||||||||||||||||||||
| CAS NO: | 7775-14-6 | |||||||||||||||||||||||||||||||||||||||||
| EC NO: | 231-890-0 | |||||||||||||||||||||||||||||||||||||||||
| Molecular Formula: | Na2O4S2 | |||||||||||||||||||||||||||||||||||||||||
| Molecular Weight: | 174.1071 | |||||||||||||||||||||||||||||||||||||||||
| Specification: | ||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1/2Na.H2O4S2/c;;1-5(2)6(3)4/h;;(H,1,2)(H,3,4)/q2*+1;/p-2 | |||||||||||||||||||||||||||||||||||||||||
| Product description:
Alias: sodium dithionite Molecular formula: Na2S2O4 English name: Sodium Hydrosulphite, Sodium dithionite Appearance: white crystalline powder Relative density (water=1): 1.02 UN No.: 1384 Chemical stability: according to the recommended disposal and storage method, it is stable at normal temperature in a closed container. Possibility of dangerous reaction: it can burn when heated or exposed to open flame. Exposed to the air will be oxidized and deteriorated. In contact with water, acids or organic substances and oxidants, it can release a lot of heat and cause severe combustion. Conditions to avoid: self-heating can occur. Incompatible substances and materials: strong oxidants, etc. Hazardous decomposition products: combustion products include sulfur oxides (SOx). Use: This product is used as a dyeing aid for cotton fabrics and a bleaching agent for cotton and wool fabrics. It is also used in medicine, mineral processing, copper plate printing, synthesis of thiourea and other sulfides. Paper industry is also used as bleach, and food-grade products are used as bleach, preservative and antioxidant
|
||||||||||||||||||||||||||||||||||||||||||
| Synonyms: | Sodium dithionite hydrate;Sodium hydrosulphite;Sodium dithionite;disodium dithionite; | |||||||||||||||||||||||||||||||||||||||||
| Molecular Structure: |
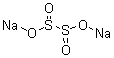 |
|||||||||||||||||||||||||||||||||||||||||