Secnil 3366-95-8
-
- Category :
Pharmaceuticals and Biochemicals/Antibiotic and antimicrobial agents
- CAS NO : 3366-95-8
- EC NO : 222-134-0
- Molecular Formula : C7H11N3O3
- Main Specifications : Secnil 3366-95-8
- Synonyms : alpha,2-dimethyl-5-nitro-1h-imidazole-1-ethanol;1-(2-methyl-5-nitro-1H-imidazol-1-yl)propan-2-ol;
Package: 10kg/drum
Uses : Antianaerobic-microbacterial drug,antitrichomonal agent
Molecular Structure:
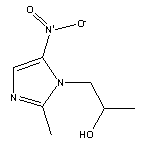
Product description:
Product Name: Secnidazole
Other names: Flagentyl, Sindose, Secnil
Category: pharmaceutical raw material bulk drugs api
CAS NO: 3366-95-8
M.F.: C7H11N3O3.1/2H20
Quality standard: YBH12562008;
Edition: CP
Applicable category:Antianaerobic-microbacterial drug,antitrichomonal agent ;
Finished dosage: Secnidazole capsules, Secnidazole tablets ;
Appearance: almost white to pale yellow crystalline powder,odourless,bitter flavor;
Freely soluble in ethanol, chloroform and propanol,sparingly soluble in 0.1mol/l hydrochloric acid, slightly soluble in water;
Assay: not less than 99% on anhydrous basis;
Loss on drying: 4.5 %~6%
Appearance of solution: clear and colorless;
Melting point: 74~78℃;
Related impurities:The peak area of total impurities ≤1%;
Heavy metal: ≤10 ppm;
Packing: 10 kg/drum
Advantage: High purity, Low heavy metal, Low related impurities, GMP certificate, Profession
Activity & Sevice: pharmaceuticals looking for distributors, wholesale pharmaceutical generic drugs, provide lab testing service pharmaceutical
Product Introduction: Secnidazole is a nitroimidazole anti-infective. Effectiveness in the treatment of dientamoebiasis has been reported.It has also been tested against Atopobium vaginae.
Secnidazole is structurally related to the commonly used 5-nitroimidazoles metronidazole and tinidazole. These drugs share a common spectrum of activity against anaerobic micro-organisms and they appear particularly effective in the treatment of amoebiasis, giardiasis, trichomoniasis and bacterial vaginosis. Secnidazole is rapidly and completely absorbed after oral administration and has a longer terminal elimination half-life (approximately 17 to 29 hours) than commonly used drugs in this class. In patients with intestinal amoebiasis or giardiasis, clinical or parasistological cure rates of 80 to 100% are achieved after treatment with a single dose of secnidazole 2 g (30 mg/kg in children), similar to the response rates achieved with multiple dosage regimens of metronidazole or tinidazole. Patients with hepatic amoebiasis appears to respond well to 5- to 7-day therapy with secnidazole, but the efficacy of this drug regimen requires further evaluation in larger numbers of patients. After administration of a single dose of secnidazole, parasitological eradication was achieved in approximately 92 to 100% of patients with urogenital trichomoniasis. Patients with bacteria vaginosis respond at least as well to a single dose of secnidazole as to single-dose tinidazole, or single- or 7-day treatment with metronidazole; clinical improvement and/or microbiological evidence of cure was attained in approximately 59 to 96% of patients. In the clinical trials reviewed, secnidazole was well tolerated; most adverse events were gastrointestinal in nature and did not require treatment intervention or withdrawal from therapy.
In summary, available evidence suggests that secnidazole is as efficacious as other 5-nitroimidazole drugs in the treatment of protozoal infections and bacterial vaginosis. The convenience and ease of administration associated with single-dose therapy, combined with a good tolerability profile, make secnidazole a suitable option to other single-dose treatments and an attractive alternative to multiple dosage regimens with other drugs in this class.
