valproate sodium
-
- Category :
Pharmaceuticals and Biochemicals/Central nervous system depressants and stimulants
- CAS NO : 1069-66-5
- EC NO : 213-961-8
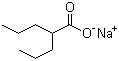
- Molecular Formula : C8H15NaO2
- Main Specifications : valproate sodium
- Synonyms : sodium 2-propylpentanoate;sodium valproate;Valproate Sodium;
Package: 10kg/drum
Uses : Antiepileptic drug
Molecular Structure:
Product description:
Product Name: Sodium Valproate
Other names: valproate sodium
Category: pharmaceutical raw material bulk drugs api
CAS NO: 1069-66-5
M.F.: C8H15NaO2
Quality standard: YBH23482006
Edition:CP
Applicable category: Antiepileptic drug;
Finished dosage: Sodium Valproate tablet;
Appearance: white crystalline powder or kernel, mildly astringent flavor, strong hygroscopicity;
Very freely soluble in water, freely soluble in methanol and ethanol, practically insoluble in acetone;
Assay:not less than 99% on the anhydrous basis;
Loss on drying: 3%
Appearance of solution: clear and colorless;
PH: 7.5~9
Related impurities: The peak area of total impurities ≤1%;
Heavy metal: ≤20ppm;
Packing: 10 kg/drum
Advantage: High purity, Low heavy metal, Low related impurities, GMP certificate, Profession
Activity & Sevice: pharmaceuticals looking for distributors, wholesale pharmaceutical generic drugs, provide lab testing service pharmaceutical
Product Introduction: Sodium valproate (INN) or valproate sodium (USAN) is the sodium salt of valproic acid and is an anticonvulsant used in the treatment of epilepsy and bipolar disorder, as well as other psychiatric conditions requiring the administration of a mood stabilizer. Sodium valproate can be used to control acute episodes of mania. Side effects can include tiredness, tremors, nausea, vomiting and sedation.[1] The intravenous formulations are used when oral administration is not possible.
In pregnancy, valproate has the highest risk of birth defects of any of the commonly-used antiepilepsy drugs. However, some epilepsy can only be controlled by valproate, and seizures also pose grave risk to mother and child.
