6-Methylcoumarin
-
- Category :
Fragrances and Aroma chemicals
- CAS NO : 92-48-8
- EC NO : 202-158-8
- Molecular Formula : C10H8O2
- Main Specifications : 99%min
- Synonyms : Methyl coumarin;2H-1-Benzopyran-2-one, 6-methyl-;5-17-10-00168 (Beilstein Handbook Reference);5-Methyl-2-hydroxyphenylpropenoic acid lactone;6-MC;6-Methyl-1,2-benzopyrone;6-Methyl-2H-1-benzopyran-2-one;6-Methyl-cis-O-coumarinic lactone;6-Methylbenzopyrone;6-Methylcoumarinic anhydride;Cocodescol;Coumarin, 6-methyl-;Pralina;Toncair;Toncarine;6-methyl coumarin;
Package: 25kg/drum
Uses : used as fragrances and flavors
Molecular Structure:
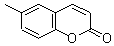
Product description:
6-Methylcoumarin Basic information
Product Name: 6-Methylcoumarin
Synonyms: 6-MC;6-Methyl-1,2-benzopyrone;6-methyl-2h-1-benzopyran-2-on;6-methyl-2H-1-Benzopyran-2-one;6-Methyl-2-oxo-2H-benzopyran;6-Methyl-2-oxochronene;6-Methyl-chromen-2-one;6-methyl-coumari
CAS: 92-48-8
MF: C10H8O2
MW: 160.17
EINECS: 202-158-8
Product Categories: Pharmaceutical Raw Materials;Coumarins;Miscellaneous;Building Blocks;Heterocyclic Building Blocks;Alphabetical Listings;Flavors and Fragrances;M-N
Mol File: 92-48-8.mol
6-Methylcoumarin Chemical Properties
mp 73-76 ℃(lit.)
bp 303 ℃ 725 mm Hg(lit.)
FEMA 2699
Fp 303℃/725mm
BRN 4222
CAS DataBase Reference 92-48-8(CAS DataBase Reference)
NIST Chemistry Reference 2H-1-Benzopyran-2-one, 6-methyl-(92-48-8)
EPA Substance Registry System 2H-1-Benzopyran- 2-one, 6-methyl-(92-48-8)
Safety Information
Hazard Codes Xn
Risk Statements 22-42/43
Safety Statements 22-36/37-45
WGK Germany 3
RTECS GN7792000
Hazardous Substances Data 92-48-8(Hazardous Substances Data)
MSDS Information
Provider Language
6-Methyl-2H-chromen-2-one English
ACROS English
SigmaAldrich English
ALFA English
6-Methylcoumarin Usage And Synthesis
Chemical Properties WHITE TO ALMOST WHITE CRYSTALLINE POWDER
General Description White crystals with a flavor of vanilla. Insoluble in water.
Air & Water Reactions Insoluble in water.
Reactivity Profile Ketones, such as 6-Methylcoumarin, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Fire Hazard 6-Methylcoumarin is combustible.
6-Methylcoumarin Preparation Products And Raw materials
Raw materials SULFUROUS ACID-->Salicylaldehyde-->Fumaric acid-->Propionic anhydride-->Sodium propionate-->Coumarin-3-carboxylic acid
