 CN
China Suppliers > AI MU >
CN
China Suppliers > AI MU > year
| 111Category: | Other Chemicals/Photography chemicals and materials |
|
|
| CAS NO: | 1314-13-2 | ||
| EC NO: | 215-222-5 | ||
| Molecular Formula: | ZnO | ||
| Molecular Weight: | 81.4084 | ||
| Specification: | |||
| InChI: | InChI=1/O.Zn/q-2;+2 | ||
| Packing: | Package: Inner is plastic bag and outer is woven bag,25kg/bag or as the client’s request | ||
| Product description: English Name: Zinc Oxide Molecular formula: ZnO Molecular weight : 81.39 Item Index Zinc oxide ( ZnO ),% 99-99.7 lead oxide ( Pb ),% ≤0.03-0.50 Oxide of manganese ( Mn ),% ≤ 0.0001-0.0005 copper oxides ( Cu ),% ≤ 0.0003-0.006 Insoluble matter in HCl,% ≤ 0.006-0.100 Loss on ignition ,% ≤ 0.2-1.0 Screen residues through(45 um mesh ) , % ≤ 0.1-0.3 Water soluble matter ,% ≤ 0.1-0.8 Volatile 105°C 0.3-0.5 Properties: Zinc oxide is white crystalline or powder; Relative density is 5.606, melting point 1975°C, it is amphoteric oxide insoluble in water, ethanol and ammonia, soluble hydroxide and ammonium chloride, while heated, it becomes yellow and resumes white after cooling down. Application: 1.Zinc oxide is used in rubber and tire industry are essential additives, are also used as natural rubber, synthetic rubber and latex vulcanization active agents and reinforcing agents, as well as coloring agents ; 2.In the chemical industry, zinc oxide is widely used as a catalyst and desulfurizer ; 3.in the coatings industry, not only zinc oxide with coloring and hiding power, but also are coatings of preservatives and brightening agent; 4. in the glass industry, zinc oxide used in specialty glass products; 5.in Ceramic Industry, zinc oxide used as a flux; 6. in printing and dyeing industry, zinc oxide used to prevent dye. |
|||
| Synonyms: | C.I. 77947;C.I. Pigment White 4;zincoxideheavy;flowers of zinc;zinc white;active zinc oxide;zinkoxyd aktiv;zinci oxidum;activox;activox b;actox14;zine oxide;zine white;zincoxide;actox16;actox216;ai3-00277;akro-zincbar85;akro-zincbar90;amalox;azo22;azo-33;azo-55;azo-55tt;azo-66;azo-66tt;ZNO;oxozinc;zinc oxygen(-2) anion; | ||
| Molecular Structure: |
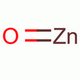 |
||