Calcium hypochlorite
-
- Category :
Inorganic chemicals
- CAS NO : 7778-54-3
- EC NO : 231-908-7
- Molecular Formula : Ca(ClO)2
- Main Specifications : Appearance and Characters: white powder, which has a strong smell; chlorine solution is yellow-green translucent liquid.Strong oxidants. Water or moist air will cause a fire explosion. Mixed with alkaline substances can cause an explosion. Exposure caused
- Synonyms : Calciumhypochloritetech;Bleaching powder;hypochlorous acid;calcium dihypochlorite;
Uses : Mainly for bleaching and textile industry cotton, hemp, silk fabric bleached pulp and paper industry, or used in rural drinking water, swimming pool water disinfection chemical industry for purification of acetylene, chloroform and other organic chemical
Molecular Structure:
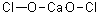
Product description:
Calcium hypochlorite, commonly known as bleach, commonly used in chemical production in the bleaching process, with its rapid onset and bleaching effect is prominent and occupy an important role in industrial production, but because it is a strong oxidant great harm to people, must not be used for other purposes outside the industry, but also pay attention to the use of self-protection, to avoid corrosion. Moreover, it also has a large number of volatile strong carcinogenicity, when you save a certain need attention.
1 Physical and Chemical Properties
Physical Properties
Appearance and Characters: white powder, which has a strong smell; chlorine solution is yellow-green translucent liquid.
Melting point (° C): 100 (decomposition)
Relative Density (water = 1): 2.35
Vapor Density (Air = 1): 6.9
Molecular formula: Ca (ClO) 2
Molecular weight: 142.99
Solubility: soluble in water.
EINECS No. 231-908-7
Chemical properties
Strong oxidants. Water or moist air will cause a fire explosion. Mixed with alkaline substances can cause an explosion. Exposure caused a risk of burning organic matter. Heat, sunlight or in case of acid will decompose emit irritating chlorine.
2. Functions and Uses
Mainly for bleaching and textile industry cotton, hemp, silk fabric bleached pulp and paper industry, or used in rural drinking water, swimming pool water disinfection chemical industry for purification of acetylene, chloroform and other organic chemical materialsmanufactured can be used for wool shrink agents, sweetening agents.
3 Cautions
HAZARDS
Health hazards: This product is dust ocular conjunctiva and respiratory irritant that can cause tooth damage to skin contact can cause severe skin damage in.
Explosion hazard: The product combustion, irritating.
First aid measures
Skin contact: Immediately remove contaminated clothing, use soap and water thoroughly washed skin doctor.
Eye contact: Did eyelid, mobile or saline irrigation water for medical treatment.
Inhalation: rapidly from the scene to fresh air and keep the airway open, such as difficulty breathing, give oxygen If not breathing, give artificial respiration immediately consult a doctor. .. ..
Ingestion: drink plenty of water, induce vomiting physician.
Fire-fighting measures
Hazardous combustion products: chloride, calcium oxide.
Fire fighting methods: firefighters are required to wear gas masks, body wear firefighting suits, fire in the wind.
Extinguishing Media: DC Water, water spray, sand.
Spill response
Emergency treatment: isolation leakage polluted area, limiting access to the proposed emergency personnel wearing a dust mask (full cover), wear protective clothing Do not direct contact with leakage rendering leakage and reducing agents, organic compounds, flammable or metal powder... contacts.
A small leak: to avoid dust, with clean shovel collection in a dry, clean, covered containers, transfer to a safe place.
Large Leak: plastic sheeting, canvas cover and then transported to collect recycling or waste disposal sites.
Handling and storage
Operation Cautions: closed operation, enhanced ventilation operator must go through specialized training, strict adherence to rules proposed operator to wear headgear electric powered air purifying respirators and dust, wear protective clothing 52,400, wear neoprene gloves away.. fire, heat, workplace smoking. away from flammable and combustible materials. avoid generating dust. Avoid reducing agents, acids in contact with. Handling of light when light unloading, packaging and containers to prevent damage. ban vibration, shock and friction. equipped appropriate variety and quantity of fire equipment and spill response equipment. Empty containers may be harmful residues.
Storage Cautions: Store in a cool, ventilated warehouse away from fire, heat storage temperature not exceeding 30 ° C, relative humidity less than 80% of the packaging seal, can not be in contact with air and reducing agents, acids, and easy (.... available) and other combustibles stored separately and avoid mixing reservoir. not a lot of storage or far. storage area should be equipped with suitable material spill.
4 Preparation
Limestone and anthracite first press 1: (0.11 to 0.13) ratio of intermittently added to the top of the lime kiln calcination temperature controlled at 800 ~ 1200 ° C, generated intermittently discharged from the end of the kiln lime, which was added water digestion, get free water containing a small amount of lime, aging more than eight days, after winnowing auger system to send to cleaners. slaked by the cyclone and powder-like gas-solid phase separation.
Containing 3% to 6% free moisture fine lime, slaked lime by the auger from the fourth position in the column chloride layer was added chlorine (chlorine liquefied gas) from the first layer through the column into the chloride, the finished bleach The first layer chlorination tower discharge.
Chemical equation is:
2Ca (OH) 2 +2 Cl2 → Ca (ClO) 2 + CaCl2 +2 H2O
Exhaust emissions from a chlorination tower, absorption generated by the caustic sodium hypochlorite, exhaust and venting.
Calcium hypochlorite preparation methods are:
Chlorine leads to calcium hydroxide solution in a high concentration of crystal Ca (ClO) 2.2 CaCl2, widely used in the bleaching of paper:
2Ca (OH) 2 + 2Cl2 → Ca (ClO) 2 + CaCl2 + 2H2O
or chlorine leads to calcium hydroxide and sodium hydroxide solution in:
2Ca (OH) 2 + 3Cl2 + 2NaOH → Ca (ClO) 2 + CaCl2 + 2H2O + 2NaCl
5 Composition / information
Hazardous components: CAS No.
Active ingredients: Ca (ClO) 2 - Calcium hypochlorite
Preparation: chlorine pass into the lime Calcium hypochlorite
2Cl2 + 2Ca (OH) 2 = CaCl2 + Ca (ClO) 2 +2 H2O
Calcium hypochlorite is chlorine and calcium hydroxide (slaked lime) was prepared by reacting, because absolutely dry calcium hydroxide does not react with chlorine, chlorine can be adsorbed by the calcium hydroxide end, based on the industrial use comprising1% or less free water to the chlorinated lime, chlorine is also used in water containing 0.06% or less. free use of these materials in water, so that the acid hydrolysis of chlorine (HClO, HCl), the resulting acid is neutralized by slaked lime. Subsequently, when the chlorination reaction depend precipitated calcium hydroxide water, the chlorine hydrolysis to proceed, so that more processes involved in the reaction of calcium hydroxide to produce a series of compounds. bleach composition of these compounds is complex.
Calcium hypochlorite easily failure if exposed in air: Ca (ClO) 2 + H2O + CO2 = CaCO3 ↓ +2 HClO
