Clopidogrel Bisulfate
-
- Category :
Pharmaceuticals and Biochemicals/Blood system agents
- CAS NO : 120202-66-6
- EC NO :
- Molecular Formula : C15H18ClNO6S2
- Main Specifications :
- Synonyms : Clopidogrel bisulphate;clopidogrel hydrogen sulphate;clopidogrel sulphate;clopidogrel bisulfate;methyl (+)-(s)-a-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4h)acetate, hydrogen sulfate salt;(2S)-(2-chlorophenyl)(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)ethanoic acid sulfate (1:1);(+/-)-Clopidogrel bisulfate;Clopidogerl Bisulfate;Clopidogrel bisulphate ��/��;Clopidogrel Bisulfate II;Clopidogrel Bisulfate I;Clopidogrel Bisulfate, FormI/FormII;Clopidogrel sulfate;Clopidogrel Hydrogensulfate;
Package: 1kg/bag. In sealed container, hidden from light.
Uses : Anticoagulants and antiplatelet aggregation agent for prevention of myocardial infarction, stroke, or has a history of peripheral arterial disease in patients with atherosclerosis.
Molecular Structure:
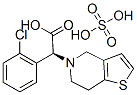
Product description:
Chemical Name:S (+) - 2 - (2 - chlorobenzene base) - 2 - (4, 7 - tetrahydrothiophene (3, 2 - c] and pyridine - 5) methyl acetate hydrogen sulfate salts
Molecular Formula :C16H16ClNO2S·H2SO4
Molecular Weight:419.91
Physical Character:White or white crystalline powder; Odourless. It is easily dissolved in water or methanol, hardly soluble in ether.
Executive standard:YBH00802013,USP
Appearance: white or almost white crystalline powder
PH: 1.5- 2.5
Residue on ignition: ≤0.1%
Loss on dry : ≤0.5%
Heavy metals: ≤0.002%
Related substance: Impurity A≤0.2%
The first Isomer of impurity B≤0.3%
Impurity C≤1.0%
Single unknown impurities≤0.1%
Total impurity≤1.5%:
Assay: 97.0% - 101.5% (calculated on dry basis)
