Aluminum chloride Suppliers, Aluminum chloride Manufacturers.
Dongying City Longxing Chemical Co., Ltd.

Aluminum chloride
Category :
Inorganic chemicalsSynonyms :
Aluminum trichloride anhydrous;Aluminum chloride;Aluminum standard concentrate 10.00 g Al;Aluminium chloride anhydrous;Aluminium trichloride;ALUMINIUM CHLORIDE HEXAHYDRATE;Aluminium chloride;Anhydrous aluminum chloride;trichloroaluminum hexahydrate;Aluminum chloride,anhydrous;CAS NO :
7446-70-0EC NO :
231-208-1Molecular Formula :
AlCl3Molecular Weight :
133.3422Main Specifications :
InChI :
InChI=1/Al.3ClH.3H/h;3*1H;;;/p-3/rAlH3.3ClH/h1H3;3*1H/p-3Product description :
Physical and chemical properties Color odor state:White crystalline powder Toxicity: low toxicity Combustibility: non-flammable Density:2.44 Volatility: easy to sublimate volatile Corrosivity: Strongly corrosive Boiling point:178℃ Stability: Easily decomposed by heat Decomposability: At normal temperature,do not decompose Melting point:194℃ Acid-base: strongly acidic Hygroscopicity::Moisture absorbent Flash point:88℃ Delixibility: easily absorb water and partially hydrolyze Solubility:Soluble in water, ethanol, acetone, ether and benzene, giving off a lot of heat Uses Aluminum trichloride is a catalyst for organic synthesis, used in medicine, pesticides, dyes, spices, plastics, lubricants, oil cracking, synthetic dyes, synthetic rubber, synthetic lubricants, synthetic detergents, spices, etc., also used in analytical reagents, preservatives, dyes, metal smelting, food grade can be used as a swelling agent, sake and other anti-color agent and pectin flocculant
Hazard category Belongs to 8.1 acid corrosion products
Fire-fighting measures Aluminum trichloride is not inherently flammable. However, when aluminum trichloride comes into contact with water, a violent reaction occurs and a large amount of heat is generated. Because it is an exothermic reaction, the higher the temperature will be, and at the same time, a lot of toxic gases will be released. Therefore, aluminum trichloride should be avoided in contact with water. In addition, in the production and use of aluminum trichloride, care should be taken to prevent contact with the moisture in the air, which will also cause a violent reaction and release toxic gases, because of this in the production and use of safety. First of all, the presence of humidity and moisture should be strictly controlled to keep the production and storage environment dry. Second, in use, aluminum trichloride should be prevented from contact with water or moisture, if accidentally contact, should be away from the scene immediately, and wear appropriate protective equipment, such as respirators, gas masks, etc. At the same time, urgent measures should be taken, such as spraying water to cool down or foam to control the scene
Emergency treatment Skin contact: Immediately remove contaminated clothing and rinse thoroughly with plenty of water
Eye contact: Immediately lift eyelids and rinse with running water for 10 minutes or with 2% sodium bicarbonate solution
Inhalation: quickly leave the scene to the fresh air, keep breathing smooth, if necessary, artificial respiration, medical treatment
Ingestion: When the patient is awake, gargle immediately and drink milk or egg white. Seek immediate medical attentionPackaging, storage, transportation Packing with PVC plastic film lined bag, external plastic bucket or woven bag,25kg/ bag, 50kg/ bag, ton bag, stored in a cool, clean, well-ventilated warehouse, away from fire heat source, must be sealed, do not get damp
Molecular Structure :
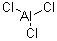
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
ChemNet is a registered trademark of Zhejiang NetSun Co., Ltd.
