Sodium hydroxide Suppliers, Sodium hydroxide Manufacturers.
Dongying City Longxing Chemical Co., Ltd.

Sodium hydroxide
Category :
Organic chemicals and DerivativesSynonyms :
Caustic soda;lye, caustic;sodium hydrate;soda lye;white caustic;caustic soda flakes;flake caustic;caustic soda solid;caustic soda pearls;solid caustic soda;Liquid Caustic Soda;Food Additives Sodium Hydroxide;Caustic soda flake;Solid Sodium hydroxide;Caustic Soda;Sodium Hydrate;Liquid CS;CAS NO :
1310-73-2EC NO :
215-185-5Molecular Formula :
NaOHMolecular Weight :
41.0045Main Specifications :
InChI :
InChI=1/Na.H2O/h;1H2/q+1;/p-1/rHNaO/c1-2/h2HProduct description :
Physical and chemical properties Color and smell state: white translucent flakes or particles Toxicity: Density: 2.13 Corrosivity: strong Boiling point: 1390℃ Hygroscopicity: strong Melting point: 318.4℃ Acidity and alkaline: strong alkaline Deliquescence: easy to deliquescence, absorb water vapor and carbon dioxide in the air to generate sodium carbonate. Solubility: It is easily soluble in water (exothermic when dissolved in water) and forms an alkaline solution. The solution has astringent and slippery taste, and is soluble in ethanol and glycerin. Uses Used in papermaking, soap, dyes, rayon, metal smelting, petroleum refining, cotton fabric finishing, coal tar product purification, and food processing, wood processing and machinery industries. Can be used for chemical experiments. In addition to being used as a reagent, it can also be used as an alkaline desiccant due to its strong water absorption and deliquescent properties. It can also absorb acid gas (for example, in the experiment of burning sulfur in oxygen, sodium hydroxide solution can be filled into a bottle to absorb toxic sulfur dioxide). Hazard category 8.2 Corrosive products Fire-fighting measures This product will not burn. It will emit heat when exposed to water and steam. Use water and sand to fight it out. However, it is necessary to prevent objects from splashing and burns when exposed to water. Emergency treatment Skin contact: Rinse with water for at least 15 minutes (dilute solution)/dry with cloth (concentrate), then wash with 5-10% magnesium sulfate or 3% boric acid solution and seek medical attention. Eye contact: Lift the eyelid immediately and wash with running water or saline for at least 15 minutes. Or rinse with 3% boric acid solution (or dilute acetic acid) and seek medical attention. Ingestion: Neutralize with vinegar, 3 to 5% acetic acid or 5% dilute hydrochloric acid, a large amount of orange juice or lemon juice when a small amount of accidental ingestion occurs; give egg white, milk or vegetable oil and seek medical attention quickly. It is prohibited to induce vomiting and gastric lavage. Packaging, storage, transportation Plastic bag or two-layer kraft paper bag (25kg/bag) outer full or middle opening steel drum (not more than 100 kg/drum), if a glass container is used to hold hot sodium hydroxide solution for a long time, the bottle cap may not be opened , It will also cause damage to the glass container.
Store in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. Inventory humidity is not more than 85%. The packaging must be sealed and kept away from moisture. It should be stored separately from easily (combustible) combustibles and acids, and avoid mixed storage. Transported as hazardous chemicals.Molecular Structure :
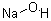
World Wide ChemNet: - International - China - Korea
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
ChemNet is a registered trademark of Zhejiang NetSun Co., Ltd.
