Trityl Candesartan Cilexetil Suppliers, Trityl Candesartan Cilexetil Manufacturers.
Taizhou Tianrui Chempharm Co., Ltd.

Trityl Candesartan Cilexetil
Category :
Intermediates/Pharmaceutical intermediatesSynonyms :
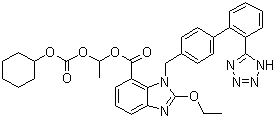
1-[[(cyclohexyloxy)carbonyl]oxy]ethyl 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1H-benzimidazole-7-carboxylate;CAS NO :
145040-37-5EC NO :
Molecular Formula :
C33H34N6O6Molecular Weight :
610.66Main Specifications :
USPInChI :
InChI=1/C33H34N6O6/c1-2-42-32-34-28-14-8-13-27(31(40)43-19-20-44-33(41)45-24-9-4-3-5-10-24)29(28)39(32)21-22-15-17-23(18-16-22)25-11-6-7-12-26(25)30-35-37-38-36-30/h6-8,11-18,24H,2-5,9-10,19-21H2,1H3,(H,35,36,37,38)Packing :
25kg/drumProduct description :
Quick Details: Product Name: Trityl Candesartan Cilexetil CAS No.: 145040-37-5 MF: C33H34N6O6 MW: 610.66 Specification: CP, USP Appearance: White to off-white crystalline powder. Usage: Ester prodrug; hydrolized in vivo to the active carboxylic acid. Used in treatment of congestive heart failure. Antihypertensive. Description: Candesartan Cilexetil is a synthetic, benzimidazole-derived angiotensin II receptor antagonist prodrug with antihypertensive activity. After hydrolysis of candesartan cilexetil to candesartan during gastrointestinal absorption, candesartan selectively competes with angiotensin II for the binding of the angiotensin II receptor subtype 1 (AT1) in vascular smooth muscle, blocking angiotensin II-mediated vasoconstriction and inducing vasodilatation. In addition, antagonism of AT1 in the adrenal gland inhibits angiotensin II-stimulated aldosterone synthesis and secretion by the adrenal cortex; sodium and water excretion increase, followed by a reduction in plasma volume and blood pressure. Patent Disclaimer: 1. Products protected by valid patents are not offered for sale in jurisdictions where the sale of such products constitutes a patent infringement. The current list only reflects the products and technologies that are available, under development: note that some products may be developed or produced for internal and experimental uses with no commercal aim. 2. Sales of products are limited to those allowed by Chapter VII PLPRC 63, the above includes Research and development quantities. 3. R&D use in accordance with (1) 35 USC 271(e)+A13(1) in the U.S.; (2) Section 69.1 of Japanese Patent Law in Japan; (3) Section 11, No. 2 of the German Patent Act of 1981 in Germany; (iv) Section 60, Paragraph 5b of the U.K. Patents Act of 1977 in the U.K.; (4) Sections 55.2(1) and 55.2(6) and other common law exemptions of Canadian patent law; (5) Section 68B of the Patents Act of 1953 in New Zealand together with the amendment of same by the Statutes Amendment Bill of 2002; (6) such related legislation and/or case law as may be or become applicable in the aforementioned countries; and (7) such similar laws and rules as may apply in various other countries. Specification: Appearance white to off white powder Conforms Solubility Slightly soluble in methanol,practically insoluble in water. Conforms Identification IR spectrum matches reference spectrum Conforms The retention time of the major peak obtained in the related substances corresponds to that of the reference Water ≤0.3% 0.1% Residue on Ignition ≤0.1% <0.1% Heavy Metals ≤0.001% <0.001% Related Substances (HPLC) Impurity A ≤0.2% 0.03% Impurity B ≤0.3% 0.03% Impurity E ≤0.2% 0.06% Any other unknown impurity ≤0.1%Molecular Structure :
World Wide ChemNet: - International - China - Korea
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
ChemNet is a registered trademark of Zhejiang NetSun Co., Ltd.
