Vonoprazan Fumarate Suppliers, Vonoprazan Fumarate Manufacturers.
Sinoway Industrial Co.LTD.

Vonoprazan Fumarate
Category :
Pharmaceuticals and BiochemicalsSynonyms :
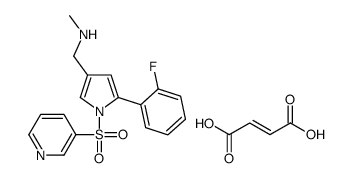
Vonoprazan fumarate;TAK-438;TAK 438;5-(2-Fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-1H-pyrrole-3-methanamine (2E)-2-butenedioate;Vonaprazan;TAK438;CAS NO :
1260141-27-2EC NO :
Molecular Formula :
C21H20FN3O6SMolecular Weight :
461.46300Main Specifications :
99% upPacking :
25kg/drumProduct description :
Function of Vonoprazan Fumarate 1) Prevention of duodenal ulcer and gastric ulcer recurrence A randomized, double-blind, multi-center clinical phase 3 trial comparing the effects of Voronazan fumarate (10mgqd and 20mgqd) and lansoprazole (15mgqd) on nonsteroidal anti-inflammatory drug-related peptic ulcer A total of 642 patients had been diagnosed with endoscopic peptic ulcers and needed to take non-steroidal anti-inflammatory drugs. The treatment period was 24 weeks. The primary endpoint was the proportion of duodenal ulcer and gastric ulcer recurrence at 24 weeks. 2) Corrosive esophagitis (erosiveoesophagitis, EO) In a randomized, double-blind, multi-center, dose-range clinical phase 2 trial, in patients with EO, compared with lansoprazole, Voronazan fumarate showed non-inferiority and was rated in Los Angeles as C/D grade patients showed excellent effects, and oral administration of 20 mg once daily became the clinically recommended dose for the treatment of EO. A randomized, double-blind, multi-center clinical phase 3 trial comparing the efficacy of this product (20mgqd) and lansoprazole (30mgqd) on EO, a total of 409 patients participated in the study. 3) Helicobacter pylori infection (Helicobacterpylori, Hp) A randomized, double-blind, multi-center clinical phase 3 trial comparing vonolazan fumarate (20mgbid) and lansoprazole (30mgbid), combined with amoxicillin and clarithromycin, to form a triple therapy, The effect of first-line medicine to eradicate Hp was included in a total of 650 Hp-positive patients who had had gastric ulcer or duodenal ulcer. The Hp eradication rates of this product and lansoprazole in the test group were 92.6% and 75.9%, respectively. For patients with clarithromycin resistance, the Hp eradication rates in the two test groups were 82.0% and 40.0%, respectively. Significantly superior to lansoprazole. In this trial, the first 50 patients who failed first-line treatment received second-line treatment with triple therapy of this product, amoxicillin and metronidazole, and the Hp clearance rate was 98%.Uses :
Corrosive esophagitis (erosiveoesophagitis, EO)Molecular Structure :
World Wide ChemNet: - International - China - Korea
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
ChemNet is a registered trademark of Zhejiang NetSun Co., Ltd.
