sodium carbonate Suppliers, sodium carbonate Manufacturers.
Tianjin Wolfsea International Co.,Ltd.

sodium carbonate
Category :
Inorganic chemicals/Inorganic saltsSynonyms :
Sodium carbonate anhydrous;Sodium carbonate solution;soda ash;disodium carbonate;Sodium carbonate-12C, 13C-depleted;calcined soda;Carbonic acid disodium salt;Sodium carbonate,high-purity;Sodium carbonate,dense;SODA ASH LIGHT;Anhydrous Sodium carbonate;carbonic acid, sodium salt;Carbonic acid, sodium salt;Caswell No. 752;Soda (VAN);Soda;Sodium carbonate,anhydrous;CAS NO :
497-19-8EC NO :
207-838-8;231-420-4Molecular Formula :
Na2CO3Molecular Weight :
105.99Main Specifications :
99.2%min for food、industry(light & dense)InChI :
InChI=1/CH2O3.2Na/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2Product description :
Soda ash light for industrial gradeMolecular formula: Na2CO3Cas No.: 497-19-8H.S.Code: 283620000Molecular weight: 105.99 (in accordance with the international association of relative atomic mass in 1999)Application: mainly used in chemical industry, glass-manufacturing, metallurgy, paper-manufacturing, textile, dyeing, synthetic detergent, soap, daily-use Chemical-industry, daily-use glass industry, washing powder, petrochemicals industry etc.Commodity: soda ash light for industrial gradespec.Standard (%)Result (%)Na2CO3≥99.299.45-65NaCL≤0.70.32-0.41Fe≤0.0040.00098-0.0013SO4≤0.03≤0.03Water Non-dissolved Matter%≤0.040.0098-0.010Gross gravity (g/ml)Around0.6 %Appearance: White crystal powderSolubility: Easily soluble in waterPacking: 25kg/40kg per bag, 20MT/20'ft container without palletStorage: Soda ash should be stored under cover in a cool, dry place.
Use: Soda ash is widely used in chemical industry, metallurgical, defense, medical, petroleum, glass, food, enamel, flax, papermaking, cleaning water, and textile industry, etc.
Manufacturing Process
1)Solvay process:
Solvay Process centered around a large hollow tower. At the bottom, calcium carbonate (limestone) was heated to release carbon dioxide.
CaCO3 → CaO + CO2
At the top, a concentrated solution of sodium chloride and ammonia entered the tower. As the carbon dioxide bubbled up through it, sodium bicarbonate precipitated:
NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4Cl
The sodium bicarbonate was then converted to sodium carbonate by heating it, releasing water and carbon dioxide:
2NaHCO3 → Na2CO3 + H2O + CO2
Meanwhile, the ammonia was regenerated from the ammonium chloride byproduct by heating it with lime (calcium hydroxide) left over from carbon dioxide generation:
CaO + H2O → Ca(OH)2
Ca(OH)2 + 2NH4Cl → CaCl2 + 2NH3 + 2H2O2)Combined Production Process (Hou's Process)
It is the same as the Solvay Process in the first few steps. But instead of treating the remaining solution with lime, carbon dioxide and ammonia is pumped into the solution and sodium chloride is added until it is saturated at 40 degree C. Then the solution is cooled down to 10 degree C. Ammonium chloride precipitates and its removed by filtration, the solution is recycled to produce more sodium bicarbonate. Hou's process eliminates the production of calcium chloride and the byproduct ammonium chloride can be used as a fertilizer.
Determination methods:
Determination of sodium carbonate content (GB 210.2-2004)
Reagent:
(1) Hydrochloric acid volumetric solution
(2) Bromocresol green-methyl red (indicator)Soda ash dense for industrial gradeMolecular formula: Na2CO3Cas No.: 497-19-8H.S.Code: 283620000Molecular weight: 105.99 (in accordance with the international association of relative atomic mass in 1999)Application: mainly used in chemical industry, glass-manufacturing, metallurgy, paper-manufacturing, textile, dyeing, synthetic detergent, soap, daily-use Chemical-industry, daily-use glass industry, washing powder, petrochemicals industry etc.Commodity: soda ash dense for industrial grade:spec.Standard (%)Result (%)Na2CO3≥99.299.45-99.75NaCL≤0.70.25-0.50Fe≤0.0040.00098-0.0013SO4≤0.03≤0.03Water Non-dissolved Matter%≤0.040.0098-0.010Retained on 180um sieve (%)≥7083.0Gross gravity (g/ml)≥0.9Around 1.05Appearance: White crystal powderSolubility: Easily soluble in waterPacking: 25kg/40kg per bag, 20MT/20'ft container without palletStorage: Soda ash should be stored under cover in a cool, dry place.
Use: Soda ash is widely used in chemical industry, metallurgical, defense, medical, petroleum, glass, food, enamel, flax, papermaking, cleaning water, and textile industry, etc.
Manufacturing Process
1)Solvay process:
Solvay Process centered around a large hollow tower. At the bottom, calcium carbonate (limestone) was heated to release carbon dioxide.
CaCO3 → CaO + CO2
At the top, a concentrated solution of sodium chloride and ammonia entered the tower. As the carbon dioxide bubbled up through it, sodium bicarbonate precipitated:
NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4Cl
The sodium bicarbonate was then converted to sodium carbonate by heating it, releasing water and carbon dioxide:
2NaHCO3 → Na2CO3 + H2O + CO2
Meanwhile, the ammonia was regenerated from the ammonium chloride byproduct by heating it with lime (calcium hydroxide) left over from carbon dioxide generation:
CaO + H2O → Ca(OH)2
Ca(OH)2 + 2NH4Cl → CaCl2 + 2NH3 + 2H2O2)Combined Production Process (Hou's Process)
It is the same as the Solvay Process in the first few steps. But instead of treating the remaining solution with lime, carbon dioxide and ammonia is pumped into the solution and sodium chloride is added until it is saturated at 40 degree C. Then the solution is cooled down to 10 degree C. Ammonium chloride precipitates and its removed by filtration, the solution is recycled to produce more sodium bicarbonate. Hou's process eliminates the production of calcium chloride and the byproduct ammonium chloride can be used as a fertilizer.
Determination methods:
Determination of sodium carbonate content (GB 210.2-2004)
Reagent:
(1) Hydrochloric acid volumetric solution
(2) Bromocresol green-methyl red (indicator)Soda ash for food gradeMolecular formula: Na2CO3Cas No.: 497-19-8H.S.Code: 283620000Molecular weight: 105.99 (in accordance with the international association of relative atomic mass in 1999)Application: mainly used in chemical industry, glass-manufacturing, metallurgy, paper-manufacturing, textile, dyeing, synthetic detergent, soap, daily-use Chemical-industry, daily-use glass industry, washing powder, petrochemicals industry etc.Commodity: soda ash for food Grade:spec.Standard (%)Result (%)Na2CO3≥99.299.40-99.60NaCL≤0.70.35-0.55Fe≤0.00350.00090-0.0015SO4≤0.03≤0.03Moisture Content %0.1%-0.4%0.1%-0.4%Water Insoluble Matter%≤0.030.0090-0.015Heavy Metal (As Pb) %≤0.001﹤0.001Arsenic(%)≤0.0002﹤0.0002Packing: 25kg/40kg/50kg/1,000kg per bag, 20MT/20’ft container without pallet and 17.5MT/20’ft container with pallet or as per customer’s requirementManufacturing Process1)Solvay process:Solvay Process centered around a large hollow tower. At the bottom, calcium carbonate (limestone) was heated to release carbon dioxide.CaCO3 → CaO + CO2At the top, a concentrated solution of sodium chloride and ammonia entered the tower. As the carbon dioxide bubbled up through it, sodium bicarbonate precipitated:NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4ClThe sodium bicarbonate was then converted to sodium carbonate by heating it, releasing water and carbon dioxide:2NaHCO3 → Na2CO3 + H2O + CO2Meanwhile, the ammonia was regenerated from the ammonium chloride byproduct by heating it with lime (calcium hydroxide) left over from carbon dioxide generation:CaO + H2O → Ca(OH)2Ca(OH)2 + 2NH4Cl → CaCl2 + 2NH3 + 2H2O2)Combined Production Process (Hou’s Process)It is the same as the Solvay Process in the first few steps. But instead of treating the remaining solution with lime, carbon dioxide and ammonia is pumped into the solution and sodium chloride is added until it is saturated at 40 degree C. Then the solution is cooled down to 10 degree C. Ammonium chloride precipitates and its removed by filtration, the solution is recycled to produce more sodium bicarbonate. Hou’s eliminates the production of calcium chloride and the byproduct ammonium chloride can be used as a fertilizer.Determination methods:Determination of sodium carbonate content (GB 210.2-2004)Reagent:(1) Hydrochloric acid volumetric solution(2) Bromocresol green-methyl red (indicator)Uses :
mainly used in chemical industry, glass-manufacturing, metallurgy, paper-manufacturing, textile, dyeing, synthetic detergent, soap, daily-use Chemical-industry, daily-use glass industry, washing powder, petrochemicals industry etc.Molecular Structure :
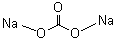
World Wide ChemNet: - International - China - Korea
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
ChemNet is a registered trademark of Zhejiang NetSun Co., Ltd.
