R134a Suppliers, R134a Manufacturers.
QUZHOU LANDE MATERIAL TRADE CO.,LTD

R134a
Category :
Organic chemicals and DerivativesSynonyms :
norflurane;FC-134a;HFC-134a;R134a;CAS NO :
811-97-2EC NO :
212-377-0Molecular Formula :
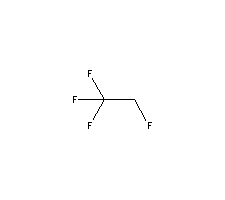
C2H2F4Molecular Weight :
102.0309Main Specifications :
ISO-TANK ,30lb/13.6KG,50lb/22.7kg,400L,800L,926L,1000LInChI :
InChI=1/C2H2F4/c3-1-2(4,5)6/h1H2Packing :
1150CYL(MAX)/20GP(30lb);640CYL/20GP(50lb);25CYL/20GP(400L);16CYL/20GP(800L);14CYL/20GP(926L);12CYL/20GP(1000L)Product description :
? 1,1,1,2-Tetrafluoroethane Chemical Properties mp -101°C bp ?26.5 °C(lit.) density 1.21 Merck 13,4734 Stability: Stable. May cause damage to the atmosphere. Incompatible with active metals, strong oxidizing agents. CAS DataBase Reference 811-97-2(CAS DataBase Reference) NIST Chemistry Reference Ethane, 1,1,1,2-tetrafluoro-(811-97-2) EPA Substance Registry System Ethane, 1,1,1,2-tetrafluoro-(811-97-2) ? Safety Information Hazard Codes Xi Safety Statements 23-38 RIDADR UN 3159 2.2 WGK Germany 1 RTECS KI8842500 Hazard Note Irritant TSCA T HazardClass 2.2 Hazardous Substances Data 811-97-2(Hazardous Substances Data) ?? 1,1,1,2-Tetrafluoroethane Usage And Synthesis Chemical Properties colourless gas or cryogenic liquid with an ether-like odour General Description A colorless gas with a slight ethereal odor. Vapors are heavier than air. Shipped liquefied under own vapor pressure. Flash point 351°F. Inhalation at high concentrations is harmful and may cause heart irregularities, unconsciousness or death without warning. Liquid contact may cause frostbite. Vapors can replace the available oxygen. Air & Water Reactions Insoluble in water. Reactivity Profile 1,1,1,2-Tetrafluoroethane is chemically inert in many situations, but can react violently with strong reducing agents such as the very active metals and the active metals. Can react with strong oxidizing agents or weaker oxidizing agents under extremes of temperature. Health Hazard Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases. Fire Hazard Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket. Uses :
refrigerant for CFC-12 substituteMolecular Structure :
World Wide ChemNet: - International - China - Korea
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
ChemNet is a registered trademark of Zhejiang NetSun Co., Ltd.
