R22 Suppliers, R22 Manufacturers.
QUZHOU LANDE MATERIAL TRADE CO.,LTD

R22
Category :
Organic chemicals and DerivativesSynonyms :
Chlorodifluoromethane;Chlorofluorocarbon 22;Fluorocarbon 22;Hydrochlorofluorocarbon 22;Methane, chlorodifluoro-;Propellant 22;Algeon 22;Algofrene 22;Algofrene type 6;Arcton 22;Arcton 4;BRN 1731036;CCRIS 858;CFC 22;Daiflon 22;Difluorochloromethane;Difluoromonochloromethane;Dymel 22;Electro-CF 22;Eskimon 22;F 22;F 22 (halocarbon);FC 22;FKW 22;Flugene 22;Fluorocarbon-22;Forane 22;Forane 22 B;Freon 22;Frigen;Frigen 22;Genetron 22;HCFC 22;HCFC-22;HFA-22;HSDB 143;Haltron 22;Isceon 22;Isotron 22;Khladon 22;Monochlorodifluoromethane;R 22 (refrigerant);R-22;Racon 22;Refrigerant R 22;Ucon 22;Chlorodifluoromethane [R22] [UN1018] [Nonflammable gas];R22;R22 [UN1018] [Nonflammable gas];UN1018;R22;CAS NO :
75-45-6EC NO :
200-871-9Molecular Formula :
CHClF2Molecular Weight :
86.4684Main Specifications :
ISO-TANK,30lb/13.6kg,50lb/22.7kg, 400L ,800L ,926L,1000LInChI :
InChI=1/CHClF2/c2-1(3)4/h1HPacking :
1150CYL(MAX)/20GP(30lb);640CYL/20GP(50lb);25CYL/20GP(400L);16CYL/20GP(800L);14CYL/20GP(926L);12CYL/20GP(1000L)Product description :
Difluorochloromethane Chemical Properties mp -146°C bp -40.8°C density 1,18 g/cm3 Water Solubility Slightly soluble Stability: Stable. CAS DataBase Reference 75-45-6(CAS DataBase Reference) NIST Chemistry Reference Methane, chlorodifluoro-(75-45-6) EPA Substance Registry System Methane, chlorodifluoro-(75-45-6) Safety Information Hazard Codes F Risk Statements 40-59 Safety Statements 23-59 RIDADR 1018 Hazard Note Non-Flammable HazardClass 2.2 Hazardous Substances Data 75-45-6(Hazardous Substances Data) Difluorochloromethane Usage And Synthesis Chemical Properties colourless gas General Description Difluorochloromethane is a colorless gas with an ethereal odor. Difluorochloromethane is shipped as a liquefied gas under its own vapor pressure. Difluorochloromethane is noncombustible. Difluorochloromethane can asphyxiate by the displacement of air. Contact with the liquid can cause frostbite. Toxic gases can be produced in fires involving Difluorochloromethane. Exposure of the container to prolonged heat or fire may cause Difluorochloromethane to rupture violently and rocket. Air & Water Reactions The liquefied gas poured into water can be violently explosive. This is due to the phase transition from superheated liquid to vapor. Reactivity Profile Difluorochloromethane is incompatible with the following: Alkalis, alkaline earth metals (e.g., powdered aluminum, sodium, potassium, zinc) . Health Hazard Inhalation at greater than 10% concentration in air may cause narcosis. Liquid may cause frostbite. Fire Hazard Special Hazards of Combustion Products: Decomposition gases are toxic and irritating. Uses :
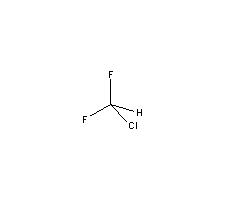
Refrigerant,PTFE material,Halon 1211 intermediateMolecular Structure :
World Wide ChemNet: - International - China - Korea
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
About Us - Top Products- Partner with Us - Contact Us - Help - Sitemap
ChemNet is a registered trademark of Zhejiang NetSun Co., Ltd.
