Ammonium Bicarbonate
Inquiry
| Post Date: | May 22,2016 | ||||||||||||||
| Expiry Date: | Nov 18,2016 | ||||||||||||||
| Detailed Description: |
Cas No. :1066-33-7
Definition:Ammonium bicarbonate is formed as shown below, or by passing carbon dioxide through a solution of the normal compound, when it is deposited as a white powder, which has no smell and is only slightly soluble in water. The aqueous solution of this salt liberates carbon dioxide on exposure to air or on heating, and becomes alkaline in reaction. The aqueous solutions of all the carbonates when boiled undergo decomposition with liberation of carbon dioxide and the substance with which the carbonate ion reacted to form the bicarbonate, in this case, ammonia: NH4HCO3 → NH3 + H2O + CO2
|
| CAS Registry Number: | 1066-33-7 |
| Synonyms: | ;Ammonium hydrogen carbonate;acid ammonium carbonate;Ammonium acid carbonate;Carbonic Acid, Monoammonium Salt;ammonium zinc carbonate (2:2:3);Ammoninum Bicarbonate;Ammonium hydrogenocarbonate; |
| Molecular Formula: | NH4HCO3 |
| Molecular Weight: | 79.05 |
| Molecular Structure: | 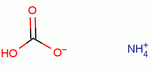
|
| Hazard Symbols: |  Xi:Irritant; Xi:Irritant; |
| Risk Codes: | R36/37/38:; |
| Safety Description: | S22:不要吸入粉尘; |
| Company: | Zibo Saibo Import & Export Co., Ltd [ China ] |
| Contact: | Ms Chen |
| Tel: | 0533-5201430 |
| Fax: | 0533-5202046 |
| Email: | manager@sinosaibo.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.