Home > Offer to Sell > Pharmaceuticals and Biochemicals > Others > Anti Estrogen Muscle Building Bulking Cycle Steroids 107868-30-4 Exemestane No Side Effect
Anti Estrogen Muscle Building Bulking Cycle Steroids 107868-30-4 Exemestane No Side Effect
Inquiry
| Post Date: | Jan 20,2017 |
| Expiry Date: | Jul 19,2017 |
| Detailed Description: |
Cas No. :107868-30-4
Quantity: 1000Kilograms Specs:99% Price:1 USD Kilograms Payment Method: Within 24 hours after we confirm the payment Anti Estrogen Muscle Building Bulking Cycle Steroids 107868-30-4 Exemestane No Side Effect Any problems, please feel free to contact me: Email:jasmine@ycgmp.com whatsapp:+8618872220697 Basic Info Product name: Exemestane Other name: Aromasin CAS:107868-30-4 Alias: Aromasin ; Exemestan;Exemestane Assay: 99% MF: C20H24O2 MW:296.40. Appearance: White or Almost White Crystalline Powder Product Category: Pharmaceutical raw materials and intermediates Packing : 25kg / barrel Certificates:ISO 9001,SGS Provide Samples: Yes. Delivery time: Within 12 hours upon receipt of payment Shipment Way: EMS, Fedex, DHL, TNT, UPS Payment method: T/T,western union, money gram, bitcoins. Storage: Shading, confined preservation Usage: Developed to help fight breast cancer, Aromasin is one of the most powerful estrogen suppressing compounds Other drugs in same class:Anastrozole,Letrozole,Tamoxifen citrate,Exemestane. Application: Function: Exemestane is an oral steroidal aromatase inhibitor that is used in ER-positive breast cancer in addition to surgery and/or radiation in post-menopausal women. The main source of estrogen is the ovaries in premenopausal women, while in post-menopausal women most of the body\'s estrogen is produced via the conversion of androgens into estrogen by the aromatase enzyme in the peripheral tissues (i.e. adipose tissue like that of the breast) and a number of sites in the brain. Estrogen is produced locally via the actions of the aromatase enzyme in these peripheral tissues where it acts locally. Any circulating estrogen in post-menopausal women as well as men is the result of estrogen escaping local metabolism and entering the circulatory system. Exemestane is an irreversible, steroidal aromatase inactivator, structurally related to the natural substrate androstenedione. It acts as a false substrate for the aromatase enzyme, and is processed to an intermediate that binds irreversibly to the active site of the enzyme causing its inactivation, an effect also known as "suicide inhibition." By being structurally similar to enzyme targets, Exemestane permanently binds to the enzymes, preventing them from converting androgen into estrogen. The estrogen suppression rate for exemestane varies from 35% for estradiol (E2) to 70% for estrone (E1). Clinical uses: Exemestane is indicated for the adjuvant treatment of postmenopausal women with estrogen-receptor positive early breast cancer who have received two to three years of tamoxifen and are switched to it for completion of a total of five consecutive years of adjuvant hormonal therapy. US FDA approval was in October 2005. Exemestane is indicated for the treatment of advanced breast cancer in postmenopausal women whose disease has progressed following tamoxifen therapy. A Phase III trial has been reported which concluded that the use of exemestane in postmenopausal women at an increased risk for breast cancer reduced the incidence of invasive breast cancer. In 4,560 women, after 35 months, the administration of exemestane at a dose of 25 mg/day resulted in a 65% reduction in the risk of breast cancer compared with placebo; annual incidence rates were 0.19% and 0.55%, respectively (hazard ratio: 0.35; 95% CI [0.18-0.70]; p = 0.002). Therapeutic Efficacy: Oral exemestane 25 mg/day for 2-3 years of adjuvant therapy was generally more effective than 5 years of continuous adjuvant tamoxifen in the treatment of postmenopausal women with early-stage estrogen receptor-positive/unknown receptor status breast in a large well-designed trial. Preliminary data from the open-label TEAM trial comparing exemestane with tamoxifen indicates that exemestane 25 mg/day is also effective in the primary adjuvant treatment of early-stage breast cancer in postmenopausal women. Interim phase III trial results in 2011 show that adding Afinitor (everolimus) to exemestane therapy against advanced breast cancer can significantly improve progression-free survival compared with exemestane therapy alone. |
| CAS Registry Number: | 107868-30-4 |
| Synonyms: | ;Exemestan;6-Methylenandrosta-1,4-diene-3,17-dione;10,13-Dimethyl-6-methylidene-7,8,9,10,11,12,13,14,15,16-decahydrocyclopenta[a]phenanthrene-;6-methylideneandrosta-1,4-diene-3,17-dione;(8ξ,9ξ,14ξ)-6-methylideneandrosta-1,4-diene-3,17-dione;Exemestane(Aromasin);Exemestone; |
| Molecular Formula: | C20H24O2 |
| Molecular Weight: | 296.4034 |
| Molecular Structure: | 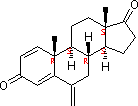
|
| Company: | Wuhan Yuancheng Gongchuang Technology Co., Ltd [ China ] |
| Contact: | Ann |
| Tel: | 8618872220697 |
| Fax: | 8618872220697 |
| Email: | jasmine@ycgmp.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


