Home > Offer to Sell > Intermediates > Pharmaceutical intermediates > CAS 56-95-1 Chlorhexidine Acetate (jerryzhang001@chembj.com)
CAS 56-95-1 Chlorhexidine Acetate (jerryzhang001@chembj.com)
Inquiry
| Post Date: | Mar 24,2017 |
| Expiry Date: | Mar 24,2018 |
| Detailed Description: |
Cas No. :56-95-1
CAS 56-95-1 Chlorhexidine Acetate
Product Name:Chlorhexidine acetate Synonym:hlorhexidine diacetate hydrate,Chlorhex,Hibitane CAS: 56-95-1 MF: C26H38Cl2N10O4 MW: 625.55 EINECS: 200-302-4 Assay: 99% Appearance: White crystalline powder Chemical Property: Melting point 154-155 Centigrade. Chlorhexidine is an antibacterial used as an antiseptic and for other applications. It is a cationic polybiguanide (bisbiguanide). It is used primarily as its salts (e.g., the dihydrochloride, diacetate, and digluconate). Usage 1. Disinfectant. The bactericidal effect of gram positive bacteria, Gram-negative bacteria and fungi are strong, are also effective against Pseudomonas aeruginosa. Used to disinfect the skin, wash the wound, and can also be used for disinfection of equipment operation. 2. For external use broad-spectrum antibacterial, antiseptic, antibacterial, sterilization and strong ability against gram negative and gram positive bacteria. 3.Chlorhexidine is used in disinfectants (disinfection of the skin and hands), cosmetics (additive to creams, toothpaste, deodorants, and antiperspirants), and pharmaceutical products (preservative in eye drops, active substance in wound dressings and antiseptic mouthwashes) Antiseptic At physiologic pH, chlorhexidine salts dissociate and release the positively charged chlorhexidine cation. The bactericidal effect is a result of the binding of this cationic molecule to negatively charged bacterial cell walls. At low concentrations of chlorhexidine, this results in a bacteriostatic effect; at high concentrations, membrane disruption results in cell death. Dental use Chlorhexidine is often used as an active ingredient in mouthwash designed to reduce dental plaque and oral bacteria. It has been shown to have an immediate bactericidal action and a prolonged bacteriostatic action due to adsorption onto the pellicle-coated enamel surface.If it is not deactivated, chlorhexidine lasts longer in the mouth than other mouthwashes, which is partly why it is to be preferred over other treatments for gingivitis.To treat periodontal pockets equal or greater than 5 mm, chlorhexidine is also available in high concentration (36%) in a gelatin chip. |
| CAS Registry Number: | 56-95-1 |
| Synonyms: | ;Chlorhexidine di(acetate);1,6-Bis(N5-[p-chlorophenyl]-N1-biguanido)hexane;Hibitane diacetate;Chlorhexidine di acetate;;2,2'-hexane-1,6-diylbis(1-{(E)-amino[(4-chlorophenyl)amino]methylidene}guanidine) acetate (1:2);Chlorhexidine diacetate; |
| Molecular Formula: | C22H30Cl2N10·2(C2H4O2); |
| Molecular Weight: | 625.55 |
| Molecular Structure: | 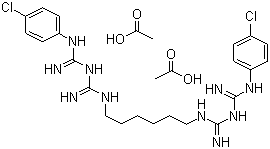
|
| Hazard Symbols: |  Xn:Harmful; Xn:Harmful; |
| Risk Codes: | R22:; R43:; |
| Safety Description: | S36/37:; |
| Company: | Nanjing Bangnuo Biotechnology Co., Ltd. [ China ] |
| Contact: | Jerry Zhang |
| Tel: | 86-25-52198306 |
| Fax: | 86-25-52198306 |
| Email: | jerryzhang001@chembj.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


