Cefotaxime sodium
Inquiry
| Post Date: | Jun 25,2015 |
| Expiry Date: | Jun 24,2016 |
| Detailed Description: |
Cas No. :64485-93-4
Cefotaxime sodium
CAS: 64485-93-4 MF: C16H16N5NaO7S2 MW: 477.45 EINECS: 264-915-9 Mp 162-163 C Refractive index 61 ° (C=1, H2O) Storage temp. 2-8° C Solubility H2O: Aqueous solutions of pH 4.3-6.2 are stable up to 3 weeks at 2-8 ° C. Soluble Form powder Color White to pale yellow crystalline powder Stability: Stable. Incompatible with strong oxidizing agents. Usage antibacterial Usage Broad spectrum third generation cephalosporin antibiotic. The name Cefotaxime applies to the isomer having a syn-methoxy imino group Synonyms: CEFOTAXIME NA SALT; CEFOTAXIME SODIUM; CEFOTAXIME SODIUM SALT; CEFOTAXIM SODIUM SALT; CLAFORAN; Ctx; 7-[2-(2-AMINO-4-THIAZOLYL)GLYOXYLAMIDO]-3-(HYDROXYMETHYL)-8-OXO-5-THIA-1-AZABICYCLO[4.2.0]OCT-2-ENE-2-CARBOXYLATE 7(2)-(Z)-(O-METHYLOXIME) ACETATE, NA; [6R-[6alpha, 7beta(z)]]-3-[(Acetyloxy)methyl]-[[(2-amine-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4, 2, 0]oct-2-ene-2-carboxylic acid monosodium salt Product Categories: API; Antibiotics for Research and Experimental Use; Beta-Lactams (Antibiotics for Research and Experimental Use); Biochemistry; Antibiotic Explorer; Heterocycles; Intermediates & Fine Chemicals; Pharmaceuticals; Sulfur & Selenium Compounds; Pharmaceutical intermediate; CLAFORAN; Antibiotic Brand names Claforan About your treatment Your doctor has ordered cefotaxime, an antibiotic, to help treat your infection. The drug will be either injected into a large muscle (such as your buttock or hip) or added to an intravenous fluid that will drip through a needle or catheter placed in your vein for 30 minutes, two to four times a day. Cefotaxime eliminates bacteria that cause many kinds of infections, including lung, skin, bone, joint, stomach, blood, gynecological, and urinary tract infections. This medication is sometimes prescribed for other uses; ask your doctor or pharmacist for more information. Pharmacology Inhibits mucopeptide synthesis in bacterial cell wall. Metabolism Cefotaxime is metabolized to desacetyl derivative (active). Elimination About 60% is recovered in the urine in 6 h; about 20% to 36% is excreted as unchanged cefotaxime and 15% to 25% as desacetyl derivative. The t ½ is 60 min (adults); 3 to 5 h (infants). Uses Cefotaxime is an antibiotic used to treat a wide variety of bacterial infections. This medication is known as a cephalosporin antibiotic. It works by stopping the growth of bacteria. How to use cefotaxime injection This medication is given by injection into a muscle or vein as directed by your doctor. If given by injection into a vein, inject the drug slowly over at least 3 minutes to avoid possible serious side effects (such as irregular heartbeat). The dosage is based on your medical condition and response to treatment. Antibiotics work best when the amount of medicine in your body is kept at a constant level. Therefore, use this drug at evenly spaced intervals. Cefotaxime (cefotaxime for injection) for Injection USP and Dextrose Injection is a sterile, nonpyrogenic, single use, packaged combination of Cefotaxime Sodium and Dextrose Injection (diluent) in the DUPLEX sterile container. The DUPLEX Container is a flexible dual chamber container. The drug chamber is filled with sterile Cefotaxime (cefotaxime for injection) Sodium USP, a semisynthetic, broad-spectrum, cephalosporin antibiotic for parenteral administration. It is the sodium salt of 7-[2-(2-amino-4-thiazolyl) glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylate 72 (Z)-(o-methyloxime), acetate (ester). The CAS Registry Number is 64485-93-4. Dosage and Administration Infection Adults IV/IM Up to 12 g/day in divided doses (from every 4 h for septicemia to every 12 h for uncomplicated infection) usually for 7 to 10 days. IV route is preferable for severe infections. Children 1 mo to 12 yr of age (weighing less than 50 kg) IV/IM 50 to 180 mg/kg/day in 4 to 6 divided doses. Children 1 mo to 12 yr of age (weighing more than 50 kg) Usual adult dose (max, 12 g/day). Infants 1 to 4 wk of age IV 50 mg/kg every 8 h. Newborns younger than 1 wk of age IV 50 mg/kg every 12 h. Gonococcal Urethritis/Cervicitis in Men and Women Adults IM 0.5 g as single dose. Rectal Gonorrhea Adults IM 0.5 g as single dose (women); 1 g as single dose (men). Perioperative Prophylaxis Adults IV/IM 1 g 30 to 90 min prior to surgery. Cesarean Section Adults IV 1 g as soon as umbilical cord is clamped; second and third dose IV/IM at 6- and 12-h intervals after first dose. Dosage Adjustment for Renal Function Impairment Reduce dose 50% in patients with Ccr less than 20 mL/min. General Advice •For IM or IV administration only. Not for intradermal, subcutaneous, or intra-arterial administration. •IV route preferred for severe or life-threatening infections, or for patients who may be poor risks because of lowered resistance. •Follow manufacturer's guidelines for reconstitution of sterile powder with respect to diluent volume, withdrawable volume, and approximate final concentration. •For IM administration, reconstitute vials with sterile water for injection or bacteriostatic water for injection. •For IV administration, reconstitute vials with at least 10 mL sterile water for injection; reconstitute infusion bottles with 50 or 100 mL sodium chloride 0.9% injection or dextrose 5% injection. •Shake to dissolve. •Thaw premixed frozen injection at room temperature or under refrigeration (at or below 41°F). Do not force thaw by immersion in water baths or microwave irradiation. Check container for minute leaks by squeezing container firmly. Discard container if leaks are detected. Do not use plastic containers in series connections because of risk of air embolism. •Reconstituted or thawed solution should be clear and pale to light yellow in color. Do not use if discolored, cloudy, or contains particulate matter. •Intermittent IV administration: solution containing 1 or 2 g cefotaxime in 10 mL sterile water for injection can be injected over a period of 3 to 5 min. Do not administer over a period of less than 3 min. Can also be administered via infusion system over longer period of time; temporarily discontinue administration of other solutions at same site during infusion of cefotaxime. •For continuous IV infusion, add solution of cefotaxime to IV bottles containing compatible fluids (sodium chloride 0.9% injection, dextrose 5% or 10% injection, dextrose 5% and sodium chloride 0.9%, 0.45%, or 0.2% injection, Ringer's lactate solution, sodium lactate injection, invert sugar 10% solution, Travasol 8.5% amino acid injection without electrolytes. •For IM administration. Dose of 2 g may be given if dose is divided and administered at different sites. Storage/Stability Store vials and bottles of dry powder below 86°F. Protect from light and excessive temperature. Refer to manufacturer's guidelines for storage and stability recommendations for reconstituted solutions. Store premixed frozen injection in freezer capable of maintaining temperature of −4°F. Thawed solution is stable for 10 days under refrigeration (at or below 41°F) or 24 h at or below 72°F. Do not refreeze thawed antibiotics. |
| CAS Registry Number: | 64485-93-4 |
| Synonyms: | ;Cefotaxime sodium salt;Cefotaxime sodium;Claforan;sodium salt of 7-[2-(2-amino-4-thiazolyl)glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate 7 (Z)-(O-methyloxime), acetate (ester);(6r-(6alpha,7beta(z)))-3-((acetyloxy)methyl)-(((2-amine-4-thiazolyl)(methoxyimino)acetyl)amino)-8-oxo-5-thia-1-azabicyclo(4,2,0)oct-2-ene-2-carboxylic acid monosodium salt;sodium (6R,7R)-3-[(acetyloxy)methyl]-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate;5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[(acetyloxy)methyl]-7-[[(2E)-2-(2-amino-4-thiazolyl)-2-(methoxyimino)-1-oxoethyl]amino]-8-oxo-, sodium salt, (6R,7R)- (1:1);cefotaxim sodium; |
| Molecular Formula: | C16H17N5NaO7S2 |
| Molecular Weight: | 478.4547 |
| Molecular Structure: | 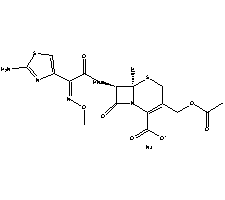
|
| Company: | Hulumimi Pharmaceuticals [ China ] |
| Contact: | Toni Zhong |
| Tel: | 027-50756080 |
| Fax: | 0086-27-88048077-794 |
| Email: | toni@ycgmp.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


