Entecavir
Inquiry
| Post Date: | Jun 06,2020 |
| Expiry Date: | Jun 06,2021 |
| Detailed Description: |
Cas No. :142217-69-4
Quantity: 1Metric Tons Specs:99%min Product Name: Entecavir Synonyms: entikawei;Entecavir USP Impurity B;Aids098045;Aids-098045;Etv;EntikaweiPian;2-Amino-9-((1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl)-3H-purin-6(9H)-one ,99.7%;Enticavir CAS: 142217-69-4 MF: C12H15N5O3 MW: 277.283 EINECS: 604-279-5 Product Categories: API;Anti-virals;Heterocycles;The latest anti hepatitis b anti-aids drugs;Inhibitors;Antiviral Agents;Nucleotides and Nucleosides;Bases & Related Reagents;Intermediates & Fine Chemicals;Nucleotides;Pharmaceuticals Mol File: 142217-69-4.mol Entecavir Structure Entecavir Chemical Properties Melting point 249-252°C Boiling point 661.4±65.0 °C(Predicted) density 1.81±0.1 g/cm3(Predicted) storage temp. -20°C Freezer form powder pka 14.22±0.60(Predicted) color white to beige optical activity [α]/D +25 to +40°, c = 0.2 in H2O CAS DataBase Reference 142217-69-4(CAS DataBase Reference) Safety Information Safety Statements 24/25 WGK Germany 3 HS Code 29339900 Hazardous Substances Data 142217-69-4(Hazardous Substances Data) MSDS Information Entecavir Usage And Synthesis Hepatitis B treatment first-line drug Entecavir is a new generation of guanine nucleoside analogues oral medicine for treatment of hepatitis B virus infection in, mainly for the treatment of adult patients with viral replication activity and serum transaminase continued to increase, or liver tissue for pathological activity of chronic hepatitis B, is currently down virus the fastest and the most powerful, the mutation rate lowest nucleoside analogues. Data show that different in patients with chronic hepatitis B, including nucleoside naive and nucleoside treated and liver cirrhosis patients, using well entecavir tablets in the treatment can control the disease rapidly and easily reach the treatment of reality end, namely the hepatitis B virus unmeasured; through adherence to treatment, a considerable portion of patients can be arrived at the end of treatment satisfaction, namely e antigen serology conversion, some patients can even reach the ideal for the treatment of end, namely surface antigen negative. mechanism of action This product is guanosine analogue, having inhibiting effect to hepatitis B virus (HBV) polymerase. It can change to the active phosphorylation by three phosphate, the half-life of three phosphate in cells is 15 hours. The natural substrate DNA guanine nucleoside and HBV polymerase three phosphate competition, entecavir three phosphate can inhibite of viral polymerase (reverse transcriptase) of all three activities: (1) HBV polymerase promoter; (2) the formation of before genomic mRNA reverse transcription negative strand; (3) HBV DNA chain is synthetised. Entecavir three phosphate on the inhibition constant HBV DNA polymerase (Ki) 0.0012 μM. entecavir three phosphate on cell entecavir α, β, δ DNA polymerase and mitochondrial DNA polymerase inhibition. Ki value is 18 to 160μM. Side Effects In the treatment of hepatitis B, entecavir as first-line antiviral drugs in the treatment of hepatitis B virus on the effect is worthy of recognition, have a significant treatment effect of serum transaminase ALT increased or liver lesions for active viral replication in patients with chronic hepatitis B in adults,. However, the clinical application of entecavir is affected due to the side effects of entecavir. The most common side effects of entecavir: the increase of ALT, fatigue, dizziness, nausea, abdominal pain, abdominal discomfort, abdominal discomfort, liver, muscle, insomnia, rubella and indigestion, also be found in neutrophils decreased slightly. These adverse reactions were mild to moderate. In trials with lamivudine controls, the incidence of adverse events is equivalent to lamivudine. According to the study, it also found that, as the same type of antiviral drugs, entecavir and the first generation of antiviral drugs have similar side effects, such as acid poisoning, hepatomegaly, liver fatty degeneration in the withdrawal will appear rebound phenomenon. In these studies, using entecavir of the patients in the treatment process, ALT increased to 10 times of the normal value upper limit and baseline values of 2 times, the generally continue to medication for a period of time, the ALT returned to normal; prior to or concurrently with viral load 2 on the numerical decline. So during the treatment, regular liver function should be tested. In the side effects of entecavir, most not easy to accept the resistance for patients, resistance will increase the difficulty of treatment, medication time to grow, for patients, psychological pressure and economic pressure will increase, half-way stopping may cause illness rebound. Although existing nucleoside analogues inhibited viral force of entecavir is strong, and mutation rate is lowest (newly diagnosed 4 years less than 1%), but the price is also the most expensive, more suitable for economic conditions, long-term treatment. Because of the side effects of entecavir, patients with hepatitis B should be taken entecavir under the guidance of specialized subject doctor, once appear uncomfortable to reflect to the doctor. In the necessary condition, timely adjustment of treatment. And the use entecavir therapy does not reduce hepatitis B through sexual contact or blood transmission of hepatitis B virus risk. Therefore, it is necessary to take appropriate protective measures. usage and dosage Patients with chronic hepatitis B should be under the guidance of experienced doctors taking this product. Recommended dosage: adult and over the age of 16 young oral this product, once a day, each 0.5 mg. lamivudine treatment viremia or patients with lamivudine resistant mutants to once a day, each 1.0 mg (0.5mg two pieces), taken on an empty stomach (a meal or a meal after at least 2 hours). Adefovir dipivoxil Adefovir dipivoxil regulates the immune system and let the immune system to attack liver cells of the intrusion of HBV , and clear the virus, but this method will develop resistance, at the same time for patients with renal dysfunction or potential renal dysfunction risk, the use of adefovir dipivoxil chronic treatment will lead to renal toxicity. If eating 1 mg every day, in the three months will produce nephrotoxicity, this will lead to severe renal failure. These patients should be closely monitoring renal function and making appropriate dosage adjustments. Bisphosphonates is quick conversion of adefovir dipivoxil in vivo, adefovir dipivoxil is a kind of single adenosine monophosphate acyclic nucleoside analogues, became to activity of the metabolites of adefovir in cellular kinases by phosphorylation in cellular kinases. A Duff Vee two phosphate through the following two ways to suppress HBV DNA polymerase (reverse transcriptase); one is with the natural substrate of deoxyadenosine triphosphate competition, the second is the integration of viral DNA and induce DNA chain elongation terminated. A Duff Vee two phosphate to HBV DNA polymerase inhibition constant (KI) is 0.1μM, but of human DNA polymeraseα and γ is weak and Ki values were 1.18μM and 0.97 μM. Anti-viral activity: in the human hepatoma cell line transfected with HBV, the concentration of A Duff Vee inhibited 50% viral DNA replication (IC50) is from 0.2 to 2.5uM. Adefovir dipivoxil applies for the treatment of hepatitis B virus replication activity and serum amino acid transfer enzyme and persistently elevated liver decompensation adult patients with chronic hepatitis B patients. Liver injury is often encountered in the course of hepatitis B treatment, hepatitis B therapy is inappropriate, as well as long-term use of drugs on the liver damage, will lead to the occurrence of liver damage. After long-term taking adefovir dipivoxil tablets, once the withdrawal will aggravate the damage of liver function. Therefore, for stopping taking the patients should be monitored for liver function. Adefovir dipivoxil is more moderate suitable for long-term use, suitable for patients with lamivudine resistant patients, their chances of producing drug resistance is small, because the amount is less, side effects are small. Entecavir is new, antiviral effect is also strong, if not treated with lamivudine, the possible resistance is the smallest, but it and lamivudine have cross resistance, if treated with lamivudine to take entecavir produced resistance rate is much greater. The above information is Chemicalbook Hanya edited. Genotoxicity In the study of reproductive toxicity, in 4 consecutive weeks of entecavir, dose up to 30mg/kg, the dosage of 90 times more than the recommended dose of 1.0mg/ day, the highest body did not find the fertility of male and female rats affected. In toxicology of entecavir studies, when dose is 35 times of body dose or more, found the dog rodent animal appeared degenerative changes. The vas deferens in the monkey experiments, no changes were found in testis. Study on reproductive toxicity in rats and rabbits, the oral dose of 200 and 13mg/kg/ day, which is equivalent to 28 times of the highest dose 1.0mg/ day (in rats) and 212 times (for rabbit), no embryo and maternal toxicity. In rabbits, the dosage of female rabbits is 883 times the daily dose of 1.0mg/ in the human body, the observed toxic effects on embryo fetal rabbit (absorption), reduce the level of ossification (tongue Bone), and the 13th rib occurrence rate increased. In the practical before birth and after birth of rats oral clinical trials converted that card quantity is greater than 94 times of people 1.0mg/ a daily dose, having not to impact on future generations. Entecavir can be from rat milk secretion. Description Entecavir is a cyclopentyl guanosine analog launched for the once-daily oral treatment of chronic hepatitis B virus (HBV) infection, and it is the third nucleoside or nucleotide analog to be marketed for this indication. Lamivudine, a deoxythiacytosine analog, and adefovir dipivoxil, a nucleotide analog, have been marketed since 1998 and 2002, respectively. Entecavir and adefovir are specifically indicated for HBV, whereas lamivudine is indicated for both HBV and HIV infections. In mammalian cells, entecavir is efficiently phosphorylated to the active triphosphate form, which competes with the natural substrate deoxyguanosine triphosphate and functionally inhibits all three activities of the HBV polymerase: (1) base priming, (2) reverse transcription of the negative strand from the pregenomic messenger RNA, and (3) synthesis of the positive strand of HBV DNA.The most common adverse events associated with the use of entecavir are similar to those typically seen with HBV therapy and include headache, abdominal pain, diarrhea, fatigue, and dizziness. Chemical Properties White to Off-White/Yellow Crystalline Powder Originator BMS (US) Uses An oral antiviral drug used in the treatment of hepatitis B infection. A guanine analogue that inhhibits all three steps in the viral replication process Definition ChEBI: Guanine substituted at the 9 position by a 4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl group. A synthetic analogue of 2'-deoxyguanosine, it is a nucleoside reverse transcriptase inhibitor with selective antiviral activity against hepatitis B virus Entecavir is phosphorylated intracellularly to the active triphosphate form, which competes with deoxyguanosine triphosphate, the natural substrate of hepatitis B virus reverse transcriptase, inhibiting every stage of the enzyme's activity, although it ha no activity against HIV. It is used for the treatment of chronic hepatitis B. Brand name Baraclude (Bristol-Myers Squibb). Pharmaceutical Applications An analog of guanosine formulated as tablets and suspension for oral use. Pharmacokinetics Oral absorption: 100% Cmax 0.5 mg/kg oral: 4.2 ng/mL Intracellular half-life: c. 16 h Volume of distribution: In excess of body water Plasma protein binding: 13% Entecavir is rapidly absorbed after administration on an empty stomach, achieving peak plasma concentrations in 1–1.5 h. Plasma steady state is achieved in 6–10 days. It is renally eliminated. Dosage adjustment is required with impaired creatinine clearance. The drug is not metabolized by cytochrome P450. No drug interactions have been identified. Clinical Use Treatment of chronic hepatitis B virus infection in patients >16 years of age Side effects Severity of adverse reactions was comparable to that of lamivudine, with headache, fatigue, upper respiratory infections and abdominal pain being most common. Lactic acidosis and hepatic steatosis were rarely observed. |
| CAS Registry Number: | 142217-69-4 |
| Synonyms: | ;2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one;2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-3,9-dihydro-6H-purin-6-one;2-amino-9-[(3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-3,9-dihydro-6H-purin-6-one;2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-1,9-dihydro-6H-purin-6-one hydrate (1:1);entecavir Baraclude; |
| Molecular Formula: | C12H15N5O3 |
| Molecular Weight: | 277.2792 |
| Molecular Structure: | 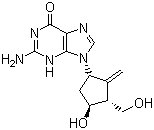
|
| Company: | shanghai missyou chemical co.,ltd. |
| Contact: | eileen zhang |
| Tel: | +86-18101936766 |
| Fax: | 021-58583907 |
| Email: | eileen@shmychem.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.