Home > Offer to Sell > Pharmaceuticals and Biochemicals > Hormones and synthetic substitutes > Eptifibatide
Eptifibatide
Inquiry
| Post Date: | Oct 09,2016 |
| Expiry Date: | Apr 07,2017 |
| Detailed Description: |
Cas No. :148031-34-9
Quantity: 1Kilograms Price:1 USD Kilograms Payment Method: T/T Product Name Eptifibatide;? Sequence? Mpr-Har-Gly-Asp-Trp-Pro-Cys-NH2 Cas No. 148031-34-9 Molecular Formula C35H49N11O9S2 Molecular Weight 832.4 Appearance White slightly yellwish powder Specific Rotation[20/D] -75.0~-95.0°(C=1,1%HAc) ? Amino Acids composition ± 10% Peptide Purity (By HPLC) ≥98% by area integration Related Substance (By HPLC) Total Impurities (%)≤2.0%? Largest Single Impurity (%)≤ 1.0% Water Content (Karl Fischer) ≤8.0% Peptide Content(N determination) ≥80% Acetate Content ≤15% IR spectrum in accordance Brief introduction: Eptifibatide (Integrilin, Millennium Pharmaceuticals, also co-promoted by Schering-Plough/Essex), is an antiplatelet drug of the glycoprotein IIb/IIIa inhibitor class.[1] Eptifibatide is a cyclic heptapeptide derived from a protein found in the venom of the southeastern pygmy rattlesnake (Sistrurus miliarius barbouri). It belongs to the class of the so-called arginin-glycin-aspartat-mimetics and reversibly binds to platelets. Eptifibatide has a short half-life. The drug is the third inhibitor of GPIIb/IIIa that has found broad acceptance after the specific antibody abciximab and the non-peptide tirofiban entered the global market. Indications Eptifibatide is used to reduce the risk of acute cardiac ischemic events (death and/or myocardial infarction) in patients with unstable angina or non-ST-segment-elevation (e.g., non-Q-wave) myocardial infarction (i.e., non-ST-segment elevation acute coronary syndromes) both in patients who are to receive non surgery (conservative) medical treatment and those undergoing percutaneous coronary intervention (PCI). The drug is usually applied together with aspirin or clopidogrel and (low molecular weight or unfractionated) heparin. Additionally, the usual supportive treatment consisting of applications of nitrates, beta-blockers, opioid analgesics and/or benzodiazepines should be employed as indicated. Angiographic evaluation and other intensive diagnostic procedures may be considered a first line task before initiating therapy with eptifibatide. The drug should exclusively be used in hospitalized patients both because of the serious degree of patients' illness and because of the possible side-effects of eptifibatide. |
| CAS Registry Number: | 148031-34-9 |
| Synonyms: | ;[3-carbamoyl-11-{4-[(diaminomethylidene)amino]butyl}-20-(1H-indol-3-ylmethyl)-1,9,12,15,18,21-hexaoxodocosahydro-7H-pyrrolo[2,1-g][1,2,5,8,11,14,17,20]dithiahexaazacyclotricosin-17-yl]acetic acid;[(1S,4R,12S,18S,21S)-12-(4-carbamimidamidobutyl)-4-carbamoyl-21-(1H-indol-3-ylmethyl)-2,10,13,16,19,22-hexaoxo-6,7-dithia-3,11,14,17,20,26-hexaazabicyclo[21.2.1]hexacos-18-yl]acetic acid;MAP-LYS-GLY-ASP-TRP-PRO-CYS-NH2;INTEGRELIN;N6-(Aminoiminomethyl)-N2-(3-mercapto-1-oxopropyl-L-lysylglycyl-L-a-aspartyl-L-tryptophyl-L-prolyl-L-cysteinamide;[(3R,11S,17S,20S,25aS)-11-(4-carbamimidamidobutyl)-3-carbamoyl-20-(1H-indol-3-ylmethyl)-1,9,12,15,18,21-hexaoxodocosahydro-7H-pyrrolo[2,1-g][1,2,5,8,11,14,17,20]dithiahexaazacyclotricosin-17-yl]acetic acid;Eptifibatide Acetate; |
| Molecular Formula: | C35H49N11O9S2 |
| Molecular Weight: | 831.96 |
| Molecular Structure: | 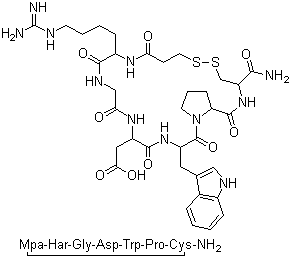
|
| Company: | Hubei Yuancheng Saichuang Technology Co.,Ltd. [ China ] |
| Contact: | fion |
| Tel: | +86-027-50755967 |
| Fax: | |
| Email: | daisy@ycphar.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


