| Detailed Description: |
Cas No. :64-18-6
Definition:Formic acid (systematically called methanoic acid) is the simplest carboxylic acid. Its formula is HCOOH or CH2O2. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings.
In nature, it is found in the stings and bites of many insects of the order Hymenoptera, mainly ants and is also present in stinging nettles. It is also a significant combustion product resulting from alternative fuelled vehicles burning methanol (and ethanol, if contaminated with water) when mixed with gasoline.[citation needed] Its name comes from the Latin word for ant, Formica, referring to its early isolation by the distillation of ant bodies. A chemical compound such as a salt from the neutralization of formic acid with a base, or an ester derived from formic acid, is referred to as Formate (or methanoate). The Formate ion has the formula HCOO−.
Packing & Loading:250KG DRUM or 35KG DRUM / 20MT
ITEM |
SPECIFICATIONS |
PURITY % |
85% min |
COLOUR INDEX |
20 max |
CHLORIDE % |
0.005 max |
SULFATE % |
0.002max |
FE % |
0.0005 max |
Evaporation Residue |
0.020 max |
|
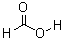
 C:Corrosive;
C:Corrosive;

