Home > Offer to Sell > Pharmaceuticals and Biochemicals > Antibiotic and antimicrobial agents > HyaPolyTM Sodium Hyaluronate injection Grade
HyaPolyTM Sodium Hyaluronate injection Grade
Inquiry
| Post Date: | Jul 10,2013 |
| Expiry Date: | Jan 06,2014 |
| Detailed Description: |
Cas No. :9067-32-7
Quantity: 100Kilograms Specs:Injection Grade Payment Method: T/T in advance Product name: HyaPolyTM Sodium Hyaluronate injection Grade Appearance: White or almost white powder or fibrous aggregate Identification: A Infrared absorption Consistent with the Ph.Eur.reference spectrum of sodium hyaluronate B Reaction Positive Assay (dried substance): 95.0%~105.0% PH: 5.0 ~ 8.5 Intrinsic viscosity: 1.6~2.2 m3/kg Nucleic acids: A260nm≤0.5 Protein ≤0.1%: Loss on drying: ≤15.0% Chlorides: ≤0.5% Iron: ≤30ppm Heavy metal: ≤10ppm Bacterial endotoxins: <0.05 IU/mg Total aerobic microbial count(TAMC): ≤100cfu/g Residual solvets(Ethanol): ≤5000ppm Storage Store in cool(2℃-10℃) away from moisture and direct sunlight. Shelf life 2 years if sealed and stored properly. |
| CAS Registry Number: | 9067-32-7 |
| Synonyms: | ;Hyaluronic acid sodium salt;HA Sodium;Hyaluronate Sodium;Oligomerie Sodium Hysluronate; |
| Molecular Formula: | C28H44N2O23·Na |
| Molecular Weight: | 799.6366 |
| Molecular Structure: | 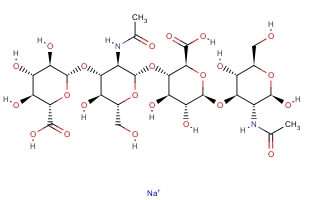
|
| Safety Description: | S22:; S24/25:; |
| Company: | Kangcare Bioindustry Co.,Ltd [ China ] |
| Contact: | Joanna SHI |
| Tel: | +86-25-83283994 |
| Fax: | +86-25-84650149 |
| Email: | joanna.shi@kangcare.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


