Home > Offer to Sell > Food and Feed additives > Food additives > L-Tyrosine (CAS: 60-18-4) (C9H11NO3)
L-Tyrosine (CAS: 60-18-4) (C9H11NO3)
Inquiry
| Post Date: | May 06,2015 |
| Expiry Date: | May 05,2016 |
| Detailed Description: |
Cas No. :60-18-4
Payment Method: T/T,Western Union,Money Gram Product Details Basic Info. Classification:Amino Acid CAS:60-18-4 Einecs:200-460-4 Rtecs:Yp2275600 Brn:392441 Pubchem:24900164 Mdl:Mfcd00002606 Appearance:White Crystals or Crystalline Powder Product Description Indication Tyrosine is claimed to act as an effective antidepressant, however results are mixed. Tyrosine has also been claimed to reduce stress and combat narcolepsy and chronic fatigue, however these claims have been refuted by some studies. Pharmacodynamics Tyrosine is a nonessential amino acid synthesized in the body from phenylalanine. Tyrosine is critical for the production of the body's proteins, enzymes and muscle tissue. Tyrosine is a precursor to the neurotransmitters norepinephrine and dopamine. It can act as a mood elevator and an anti-depressant. It may improve memory and increase mental alertness. Tyrosine aids in the production of melanin and plays a critical role in the production of thyroxin (thyroid hormones). Tyrosine deficiencies are manifested by hypothyroidism, low blood pressure and low body temperature. Supplemental tyrosine has been used to reduce stress and combat narcolepsy and chronic fatigue. Mechanism of action Tyrosine is produced in cells by hydroxylating the essential amino acid phenylalanine. This relationship is much like that between cysteine and methionine. Half of the phenylalanine required goes into the production of tyrosine; if the diet is rich in tyrosine itself, the requirements for phenylalanine are reduced by about 50%. The mechanism of L-tyrosine's antidepressant activity can be accounted for by the precursor role of L-tyrosine in the synthesis of the neurotransmitters norepinephrine and dopamine. Elevated brain norepinephrine and dopamine levels are thought to be associated with antidepressant effects. Absorption L-tyrosine is absorbed from the small intestine by a sodium-dependent active transport process. Metabolism In the liver, L-tyrosine is involved in a number of biochemical reactions, including protein synthesis and oxidative catabolic reactions. L-tyrosine that is not metabolized in the liver is distributed via the systemic circulation to the various tissues of the body. |
| CAS Registry Number: | 60-18-4 |
| Synonyms: | ;3-(4-Hydroxyphenyl)-L-alanine;H-Tyr-OH;L-tyrosine,99+% (98% ee/glc);L-tyrosine free base cell culture*tested;L-tyrosine plant cell culture tested;L-Tyrosine, Free Base;tyrosine usp;Tyr;Tyrosine,L- (8CI); (-)-a-Amino-p-hydroxyhydrocinnamicacid; (2S)-2-Amino-3-(4-hydroxyphenyl)propanoic acid;(S)-2-Amino-3-(4-hydroxyphenyl)propanoic acid; (S)-Tyrosine; (S)-a-Amino-4-hydroxybenzenepropanoicacid; 12: PN: US20090069547 PAGE: 10 claimed protein; 50: PN: WO2007067983SEQID: 199 unclaimed protein; 58: PN: US20040014159 SEQID: 33 unclaimedprotein; Benzenepropanoic acid, a-amino-4-hydroxy-, (S)-; L-(-)-Tyrosine; L-Phenylalanine, 4-hydroxy-;L-p-Tyrosine; NSC 82624; NSC 9973; Propanoic acid,2-amino-3-(4-hydroxyphenyl)-, (S)-; Tyrosine; p-Tyrosine |
| Molecular Formula: | C9H11NO3 |
| Molecular Weight: | 181.19 |
| Molecular Structure: | 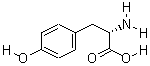
|
| Hazard Symbols: |  Xi:Irritant; Xi:Irritant; |
| Risk Codes: | R36/37/38:; |
| Safety Description: | S26:; S36:; |
| Company: | Hubei Yuancheng Saichuang Technology [ China ] |
| Contact: | aimee |
| Tel: | +86-027-50755968 |
| Fax: | +86-027-68886696 |
| Email: | avalan@chembj.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


