Home > Offer to Sell > Intermediates > Pharmaceutical intermediates > Ledipasvir (GS-5885) CAS 1256388-51-8 (jerryzhang001@chembj.com)
Ledipasvir (GS-5885) CAS 1256388-51-8 (jerryzhang001@chembj.com)
Inquiry
| Post Date: | Apr 20,2017 |
| Expiry Date: | Apr 20,2018 |
| Detailed Description: |
Cas No. :1256388-51-8
Payment Method: T/T; Western Union; Moneygram and Bitcoin Ledipasvir (GS-5885) CAS 1256388-51-8 Product Name: Ledipasvir (GS-5885) Synonyms: GS 5885;GS5885;GS-5885;Ledipasvir;GS-5885/Ledipasvir;gs-5885/gs5885;Ledipasvir / GS 5885;GS 588 CAS: 1256388-51-8 MF: C49H54F2N8O6 Molecular Weight: 889.0 Product Categories: API Categories: NS5A inhibitors;Fluorenes;Carbamates;Benzimidazoles;Cyclopropanes;Imidazoles;Breakthrough therapy Purity : 98% Solubility DMSO Storage at -20°C Appearance : White powders Package : 50g/foil bag Usage : Ledipasvir is most commonly used in combination with sofosbuvir for treatment in chronic hepatitis C genotype 1 patients. This drug has been tested and shown efficacy in treatment-naive and treatment experienced patients. Product Description : Ledipasvir (formerly GS-5885) is a drug for the treatment of hepatitis C that was developed by Gilead Sciences.After completing Phase III clinical trials, on February 10, 2014 Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin. Ledipasvir is an inhibitor of the hepatitis C virus NS5A protein. Data presented at the 20th Conference on Retroviruses and Opportunistic Infections in March 2013 showed that a triple regimen of the nucleotide analog inhibitor sofosbuvir, ledipasvir, and ribavirin produced a 12-week post-treatment sustained virological response (SVR12) rate of 100% for both treatment-naive patients and prior non-responders with HCV genotype 1.The sofosbuvir/ledipasvir coformulation is being tested with and without ribavirin. In February 2014 Gilead has filed for United States Food and Drug Administration (FDA) approval of ledipasvir/sofosbuvir oral treatment, without interferon and ribavirin. On October 10, 2014 the FDA approved the combination product ledipasvir 90 mg/sofosbuvir 400 mg called Harvoni. Application : GS-5885 is an inhibitor of the hepatitis C virus (HCV) NS5A protein and exhibits potent suppression of genotype 1 HCV replicons. GS-5885 was well tolerated and resulted in median maximal reductions in HCV RNA ranging from 2.3 log(10) IU/ml (1 mg QD) to 3.3 log(10) IU/ml (10 mg QD in genotype 1b and 30 mg QD). E(max) modeling indicated GS-5885 30 mg was associated with>95% of maximal antiviral response to HCV genotype 1a. HCV RNA reductions were generally more sustained among patients with genotype 1b vs. 1a. Three of 60 patients had a reduced response and harbored NS5A-resistant virus at baseline. NS5A sequencing identified residues 30 and 31 in genotype 1a, and 93 in genotype 1b as the predominant sites of mutation following GS-5885 dosing. Ledipasvir (formerly GS-5885) is currently in Phase III clinical trials. |
| CAS Registry Number: | 1256388-51-8 |
| Synonyms: | ;Methyl[(2S)-1-{(6S)-6-[5-(9,9-Difluoro-7-{2-[(1R,3S,4S)-2-{(2S)-2[(methoxycarbonyl)amino]-3-methylbutanoyl}-2-azabicyclo[2.2.1]-hept-3-yl]-1H-benzimidazol-6-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl}-3-methyl-1-oxobutan-2-yl]carba-mate; |
| Molecular Formula: | C49H54F2N8O6 |
| Molecular Weight: | 889.00 |
| Molecular Structure: | 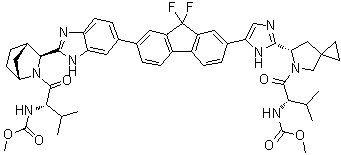
|
| Company: | Nanjing Bangnuo Biotechnology Co., Ltd. [ China ] |
| Contact: | Jerry Zhang |
| Tel: | 86-25-52198306 |
| Fax: | |
| Email: | jerryzhang001@chembj.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


