Letrozole(Femara)
Inquiry
| Post Date: | Mar 19,2015 |
| Expiry Date: | Mar 18,2016 |
| Detailed Description: |
Cas No. :112809-51-5
Payment Method: western union , money gram , paypal , bank t Letrozole(Femara) CAS No: 112809-51-5 Molecular formula: C17H11N5 Molecular weight: 285.3 Appearance: White power Assay: 99 percent Delivery time: 5-7working days door to door Minimum order: 100g Supply ability: 100kg/month Quality standard: USP32 Detail: Letrozole is approved by the United States Food and Drug Administration (FDA) for the treatment of local or metastatic breast cancer that is hormone receptor positive or has an unknown receptor status in postmenopausal women. Letrozole is intended for use only by women who are no longer of childbearing age. Possible candidates must have already ceased to menstruate. It is often used in patients who have already undergone other cancer treatments, such as radiation, chemotherapy, and used other cancer drugs, like tamoxifen. This nonsteroidal aromatase inhibitor may help prevent any remaining cancer cells from spreading. |
| CAS Registry Number: | 112809-51-5 |
| Synonyms: | ;4,4'-(1h-1,2,4-Triazol-1-Ylmethylene)Bisbenzonitrile;Lelrozol;4,4'-(1h-1,2,4-Triazol-1-Ylmethylene) Bis-Benzonitrile;Femara;Letrozol;Letrazole; |
| Molecular Formula: | C17H11N5 |
| Molecular Weight: | 285.31 |
| Molecular Structure: | 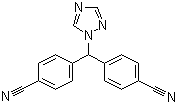
|
| Company: | Hugeraw Health Technology Co.,Ltd. [ China ] |
| Contact: | sunny |
| Tel: | +86-523-51904695 |
| Fax: | +86-523-51904698 |
| Email: | hugerawsales06@hotmail.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


