Levitra Vardenafil
Inquiry
| Post Date: | Jun 25,2015 |
| Expiry Date: | Jun 24,2016 |
| Detailed Description: |
Cas No. :224785-91-5
Levitra (Vardenafil)
Levitra (Vardenafil) is a PDE5 inhibitor which is used to treat erectile dysfunction. Vardenafil under the brand name Levitra. Action Like other PDE5 inhibitor, Sildenafil (Viagra), Vardenafil is closely related in its function to reverse impotence. Prolonged and more rigid erections and possible testosterone increase are experienced with its use. Possibly negative side effects include: gastric pain, back pain, abdominal cramping, blurred or abnormal vision, eye pain, sensitivity to light, swelling of the face, raised blood pressure, palpitations, racing of the heart, rash, and itching. Rare but serious side effects include heart attack, loss of vision in one eye and penile tissue damage. Technical Data A total of 1,057 men participated in a study, involving separate treatment using Sildenafil and Vardenafil for four weeks, with a one-week detoxification period in between. Results indicated that 38.9% preferred Vardenafil (Levitra) verses 34.5% who preferred Sildenafil (Viagra) (26.6% had no preference). Vardenafil was found to be much more potent in increase in erectile function, erection rigidity, penetration success, maintenance of erection post penetration (until orgasm), erection confidence, and overall sexual satisfaction over Viagra (1). Background Both Bayer Pharmaceuticals and GSK marketed the drug, Vardenafil under the brand name Levitra. As of 2005, the rights were transferred solely to Bayer. It comes in 2.5 mg, 5 mg, 10 mg, and 20 mg doses in round orange tablet form available through prescription. It can also be obtained through many underground labs and research companies. Action Like other PDE5 inhibitor, Sildenafil (Viagra), Vardenafil is closely related in its function to reverse impotence. Prolonged and more rigid erections and possible testosterone increase are experienced with its use. Possibly negative side effects include: gastric pain, back pain, abdominal cramping, blurred or abnormal vision, eye pain, sensitivity to light, swelling of the face, raised blood pressure, palpitations, racing of the heart, rash, and itching. Rare but serious side effects include heart attack, loss of vision in one eye and penile tissue damage. Technical Data A total of 1,057 men participated in a study, involving separate treatment using Sildenafil and Vardenafil for four weeks, with a one-week detoxification period in between. Results indicated that 38.9% preferred Vardenafil (Levitra) verses 34.5% who preferred Sildenafil (Viagra) (26.6% had no preference). Vardenafil was found to be much more potent in increase in erectile function, erection rigidity, penetration success, maintenance of erection post penetration (until orgasm), erection confidence, and overall sexual satisfaction over Viagra (1). In a 12 week double-blind study of males suffering from ED, patients received either 10 mg’s Vardenafil or a placebo group for 4 weeks, then had the option to switch to either 5 or 20 mgs (or matching placebo in control group) for four weeks, switching dosage again in the last 4 weeks was optional. Average sexual satisfaction and erection hardness rates increased with Vardenafil treatment to 43%, 59%, and 63% at weeks 4, 8, and 12, respectively. Effects reported by the placebo group were 10%, 21%, and 23%. Overall satisfaction was increased an average of 50-65% between weeks 4-12 verses 17-28% for placebo. Reduced symptoms of depression and improved self-confidence were significantly greater in the Levitra group over the placebo group (2). It was concluded through this study that Levitra is an effective medication in treating impotence (2). In a 26 week study of 800 middle aged men (mean age mid to late 50’s) who had been diagnosed with ED for greater than 3.6 years were treated with Vardenafil in 4 separate groups: 5 mg, 10 mg, 20 mg and placebo groups. Successful penetration (first attempt) was greater in all three treated groups compared to the placebo group (3). Specifically 67% in the 5-mg Vardenafil group, 77% in the 10-mg Vardenafil group, and 74% in the 20-mg Vardenafil group compared with 46% for placebo (3). Further studies of least 1 successful penetration (in 3 tries) was 82% for 5 mg, 88% for 10 mg, and 85% for 20 mg Vardenafil compared with 68% for placebo(3). Few reported negative side effects including headache (the most popular side) experienced were mild to moderate. Side effects reported ranged from 10% to 22% in the Vardenafil groups compared to 4% among patients in the placebo group (3). Vardenafil hydrochloride is the chemical name of active ingredient in Levitra. Levitra is a registered trademark of Bayer HealthCare, GlaxoSmithKline, and Schering-Plough in the United States and/or other countries. I am Toni, email adress : toni at ycgmp dot com Tel:86-18872220727 |
| CAS Registry Number: | 224785-91-5 |
| Synonyms: | 2-[2-Ethoxy-5-(4-ethylpiperazin-1-yl)sulfonylphenyl]-5-methyl-7-propyl-1H-imidazo[5,1-f][1,2,4]triazin-4-one hydrochloride;2-{2-ethoxy-5-[(4-ethylpiperazin-1-yl)sulfonyl]phenyl}-5-methyl-7-propylimidazo[5,1-f][1,2,4]triazin-4(1H)-one dihydrochloride;Vardenafil HCl; |
| Molecular Formula: | C23H34Cl2N6O4S |
| Molecular Weight: | 561.5249 |
| Molecular Structure: | 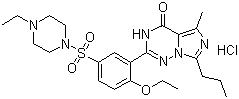
|
| Company: | Hulumimi Pharmaceuticals [ China ] |
| Contact: | Toni Zhong |
| Tel: | 86-18872220727 |
| Fax: | 0086-27-88048077-794 |
| Email: | toni@ycgmp.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


