Magnesium Hydroxide
Inquiry
| Post Date: | Apr 28,2021 | |||||||||||||||||||||||||||
| Expiry Date: | Oct 25,2021 | |||||||||||||||||||||||||||
| Detailed Description: |
Cas No. :1309-42-8
Basic Information Magnesium hydroxide, chemical formula Mg (OH) 2, Appearance is a white amorphous powder. Alias cassiterite, light burned magnesia, magnesium hydroxide in water suspension called magnesium hydroxide emulsion, referred to as magnesium milk, Product name is Magnesium hydroxide. Magnesium hydroxide is a colorless hexagonal crystal or white powder, insoluble in water and alcohol, soluble in dilute acid and ammonium salt solution, the aqueous solution was weakly alkaline. Solubility in water is small, but the water-soluble fraction is completely ionized. The concentration of saturated aqueous solution was 1.9 mg / L (18 ° C). Heat to 350 ° C to lose water to form magnesium oxide. Physical and chemical properties Appearance and Properties: Odorless white powder, Density: 2.36g / cm3, Melting point: 350ºC (Decomposes), Boiling point: 100ºC at 760mmHg, Flash point: will not burn, Stability: Stable. Magnesium hydroxide is a strong alkali (magnesium hydroxide solubility is very small, alkaline solution is weak, sometimes as a weak base treatment), heated to 623K (350 ℃) that is dehydrated, easily soluble in acid or ammonium salt solution. Like magnesia, it absorbs carbon dioxide in the air and forms a basic carbonate with a composition of 5MgO · 4CO2 · xH2O. Above 350 ° C, it decomposes to magnesia and water, but it is only dehydrated at temperatures above 1800 ° C. index
Application areas 1. Magnesium hydroxide as a flue gas desulfurization agent in the environmental protection, can replace caustic soda and lime as a waste acid neutralizer. Also used as oil additives, play a preservative and desulfurization. In addition, it can also be used in the electronics industry, medicine, sugar refining, insulation materials and other products made of magnesium. 2. Magnesium hydroxide buffer performance, reactivity, adsorption capacity, thermal decomposition properties are more excellent, both as a chemical material and intermediate, but also as a green flame retardant and additives for rubber, plastic, fiber And resin and other polymer materials in the industry. 3 can be used as a flame retardant or flame retardant filler added to polyethylene, polypropylene, polystyrene and ABS resin, a good flame retardant and smoke-suppressing effect, the amount of 40 to 20 copies. However, the surface of the particles need to be treated with anionic surfactants, the use of inexpensive high-grade fatty acid alkali metal salts or alkyl sulfates and sulfonated succinate anionic surfactant, the amount of about 3%. 4. Magnesium hydroxide is a new type of filled flame retardant, which releases the bound water by thermal decomposition and absorbs a large amount of latent heat to reduce the surface temperature of the composite material in which it is filled. It has the effect of inhibiting polymer decomposition and The resulting combustible gas cooling effect. Magnesium hydroxide is recognized as an excellent flame retardant with three functions of flame retardancy, smoke suppression and filling in the rubber and plastic industry. Widely used in rubber, chemicals, building materials, plastics and electronics, unsaturated polyester and paint, coatings and other polymer materials. Especially for the mine air duct coating cloth, PVC whole core transport belt, flame retardant aluminum panels, flame retardant tarpaulin, PVC wire and cable materials, mining cable sheath, cable accessories flame retardant, smoke and antistatic , Can replace aluminum hydroxide, with excellent flame retardant effect. Compared with similar inorganic flame retardants, magnesium hydroxide has a better smoke suppressing effect. Magnesium hydroxide in the production, use and disposal of non-hazardous substances in emissions, but also to neutralize the combustion process of acid and corrosive gases. When used alone, the amount is generally 40% to 60%. With good compatibility with the substrate resin, thermoplastic resins and rubber products are excellent flame retardants, adhesives commonly used as additive flame retardant or flame retardant filler, the reference dosage of 40 to 200 parts. In the industry, it is used in the manufacture of magnesium salt, activated magnesia, pharmaceuticals, fine ceramics, thermal insulation materials, sugar refining, flue gas desulfurization agent, oil preservative additive, acid waste water neutralizer and color television imaging cone glass coating. 5. For the manufacture of magnesium salts, sugar refining, pharmaceutical industry, household chemicals. 6. Magnesium hydroxide emulsion in medicine as antacids and laxatives. Preparation Brine and lime By the brine (MgCl2) and lime [Ca (OH) 2] reaction. The pre-purified purified brine and digested lime treated lime milk are precipitated in a sedimentation tank, the flocculant is added into the obtained slurry, mixed well, and then into the settling tank for separation, and then After filtration, washing, drying, crushing, obtained magnesium hydroxide products. The MgCl2 + Ca (OH) 2 → CaCl2 + Mg (OH) Brine - ammonia method Purification process to remove sulfate, carbon dioxide, a small amount of impurities such as boron as raw material, ammonia as a precipitating agent in the reactor for precipitation reaction, the reaction before the introduction of a certain amount of seed crystal, fully stirred. Brine and ammonia ratio of 1: (0.9 ~ 0.93), temperature control at 40 ℃. After the reaction, add flocculant, the precipitate after filtration, washing, drying, crushing, obtained magnesium hydroxide products. The MgC12 +2 NH3 · H2O → Mg (OH) 2 +2 NH4Cl This test method to be improved yield, shorten the washing cycle, improve and perfect the production process. Ling Mitu - hydrochloric acid - ammonia method Magnesite with anthracite or coke in the kiln calcined to generate magnesium oxide and carbon dioxide. Bitter soil water slurry into a predetermined concentration of hydrochloric acid reaction prepared magnesium chloride solution. The magnesium chloride solution is reacted with ammonia in a certain concentration in a reactor, and the product is washed, settled, separated by filtration, dried and pulverized to obtain the magnesium hydroxide product. According to the need to add surface treatment agent for surface treatment. Concentrated seawater extraction method In the use of concentrated seawater to extract the slurry of magnesium hydroxide, the presence of Ca2 + is an important factor affecting product purity and quality. In this paper, the pretreatment of concentrated seawater magnesium was first studied, and the reaction conditions for the removal of calcium by sodium carbonate were investigated. The effects of sodium carbonate removal, sodium carbonate addition, stirring speed, aging time and stirring time on the calcium ion removal rate and magnesium ion loss rate in concentrated seawater were studied. The optimum operating conditions were determined. Under the best operating conditions, the calcium removal rate is controlled above 60%. Which effectively reduces the calcium Assay of the product in the concentrated seawater extraction of the slurry magnesium hydroxide and fully ensures the purity of the subsequent synthetic magnesium hydroxide product. At the same time for the extraction of magnesium hydroxide product after further use of mother liquor provides low impurity conditions. Precautions Magnesium hydroxide is poorly soluble in water and is a weak base, but it is slightly irritating to the eyes, respiratory system and skin. Therefore, the use of appropriate protection should be done, wear gloves or goggles, accidentally contact with the eyes, immediately rinse with plenty of water and seek medical advice. Basically no corrosive. Acute toxicity: Oral, LD50 = 8500mg / kg (mice) |
| CAS Registry Number: | 1309-42-8 |
| Synonyms: | ;-Magnesium hydroxide, Ph.Eur.4, BP2001, USP24, E528;Magnesium hydroxide, min. 95%, 10-15 micron;Magnesium hydrate,high purity flameretardant;asahiglass200-06;baschem12;causticmagnesite;combustrol500;FR-20;magnesium dihydroxide;magnesium hydrate; |
| Molecular Formula: | Mg(OH)2 |
| Molecular Weight: | 42.3192 |
| Molecular Structure: | 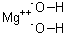
|
| Hazard Symbols: |  Xi:Irritant; Xi:Irritant; |
| Risk Codes: | R36/37/38:; |
| Safety Description: | S26:; S37/39:; |
| Company: | Shandong Swift China International Co., Ltd. [ China ] |
| Contact: | Charles Li |
| Tel: | 0536-5191398 |
| Fax: | 0536-5191398 |
| Email: | sales@xunhuachem.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


