Home > Offer to Sell > Pharmaceuticals and Biochemicals > Others > Muscle Gaining Pharmaceutical Raw Materials Powder Bodybuilding Dapoxetine 98% CAS 119356-77-3
Muscle Gaining Pharmaceutical Raw Materials Powder Bodybuilding Dapoxetine 98% CAS 119356-77-3
Inquiry
| Post Date: | Jan 20,2017 |
| Expiry Date: | Jul 19,2017 |
| Detailed Description: |
Cas No. :119356-77-3
Quantity: 1000Kilograms Specs:99% Price:1 USD FCL Payment Method: Within 24 hours after we confirm the payment Muscle Gaining Pharmaceutical Raw Materials Powder Bodybuilding Dapoxetine 98% CAS 119356-77-3 Any problems, please feel free to contact me: Email:jasmine@ycgmp.com whatsapp:+8618872220697 Quick Detail: Product Name: Dapoxetine CAS No.: 119356-77-3 M.F.: C21H23NO M.W.: 305.4134 g/mol Assay: 98% Packing: foil bag Appearance: White crystalline powder Usage: Bodybuilding , Muscle Gaining Application: Dapoxetine is a white powder substance and water-soluble. Taken 1-3 hours before sexual activity, it is rapidly absorbed in the body. Its maximum plasma concentration (Cm) is reached 1-2 hours after oral administration. Randomized, double blind, placebo-controlled trials have confirmed the efficacy of dapoxetine for the treatment of PE.Different dosage has different impacts on different type of PE. Dapoxetine 60 mg significantly improves the mean intravaginal ejaculation latency time (IELT) compared to that of dapoxetine 30 mg in men with lifelong PE, but there is no difference in men with acquired PE. Dapoxetine, given 1-3 hours before sexual episode, prolongs IELT, increases the sense of control and sexual satisfaction in men of 18 to 64 years of age with PE. Since PE is associated with personal distress, interrelationship difficulty, dapoxetine provides help for men with PE to overcome this condition. Because lack of specific approval treatment for PE in the US and some other countries, other SSRIs such as fluoxetine, paroxetine, sertraline, fluvoxamine, and citalopram have been used as off label drugs to treat PE. Waldinger’s meta analysis shows that the use of these conventional antidepressants increasing IELT from two to ninefold above base line in comparison of three to eightfold when dapoxetine is used. However, these SSRIs must be taken daily in order to achieve meaningful efficacy, and the long half-life increases the risk of the drug accumulation and as a consequence increased of adverse effects such as decreasing sexual libido and causing erectile dysfunction. Dapoxetine, on the other hand, is a fast-acting SSRI. It is rapidly absorbed and eliminated from the body within a few hours. This favorable pharmacokinetics minimizes the risk of the drug’s accumulation in the body, and therefore reducing side effects. Contraindications A contraindication is a situation in which a drug should not be used, because it may be harmful to the patient. Dapoxetine should not be used in men with moderate to severe hepatic impairment and in those receiving CYP3A4 inhibitors such as ketoconazole, ritonavir, and telithromycine. Dapoxetine can also not be used in patients with heart failure, permanent pacemaker, or other significant ischemic heart disease. Caution is advised in men receiving thioridazine, monoamine oxidase inhibitors, SSRIs, serotonin-norepinephrine reuptake inhibitors, or tricyclic antidepressant. If a patient stops taking one of these drugs, he should wait for 14 days before taking dapoxetine. If a patient stops taking dapoxetine, he should wait for 7 days before receiving these drugs. Adverse effects The most common effects when taking dapoxetine are nausea, dizziness, dry mouth, headache, diarrhea, and insomnia. Discontinuation due to adverse effects is dose related. According to McMahon in recent study in Asia, the rate of discontinuation is 0.3%, 1.7%, and 5.3% of 1067 studied subjects with placebo, dapoxetine 30 mg, and dapoxetine 60 mg respectively. Unlike others SSRIs used to treat depression, which have been associated with high incidences of sexual dysfunction, dapoxetine is associated with low rates of sexual dysfunction. Taken as needed, dapoxetine has very mild adverse effects on loss of libido (<1%) and ED (<4%). Overdose No case of the drug overdose has been reported during clinical trials. |
| CAS Registry Number: | 119356-77-3 |
| Synonyms: | ;(aS)-N,N-Dimethyl-a-[2-(1-naphthalenyloxy)ethyl]benzenemethanamine;(S)-(+)--N,N-Dimethyl-1-phenyl-3-(1-naphthalenyloxy)propanamine;(1S)-N,N-dimethyl-3-(naphthalen-1-yloxy)-1-phenylpropan-1-amine hydrochloride (1:1);(1S)-N,N-dimethyl-3-(naphthalen-1-yloxy)-1-phenylpropan-1-amine;N,N-dimethyl-3-(2-naphthyloxy)-1-phenyl-propan-1-amine;S-Dapoxetine;DL-Dapoxetine;Dapoxetine free base; |
| Molecular Formula: | C21H23NO |
| Molecular Weight: | 305.4134 |
| Molecular Structure: | 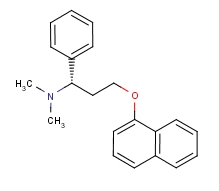
|
| Company: | Wuhan Yuancheng Gongchuang Technology Co., Ltd [ China ] |
| Contact: | Ann |
| Tel: | 8618872220697 |
| Fax: | 8618872220697 |
| Email: | jasmine@ycgmp.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.