Omeprazole
Inquiry
| Post Date: | Aug 23,2017 |
| Expiry Date: | Feb 19,2018 |
| Detailed Description: |
Cas No. :73590-58-6
CAS:73590-58-6
Name:Omeprazole Formula:C17H19N3O3S Molecular Weight:345.42 Synonyms: Pepticus;Zoltum;Lensor;Pepticum;Indurgan;Omerprazole;Omisec;Gibancer;Prazidec;Ulzol;Omezolan;Ulsen;Ortanol;Victrix;Omeprazon;Omapren;Zimor;Procelac;Dudencer;Proclor;Antra;Omesek;Omeprol;Mopral;Gasec;Peptilcer;Nopramin;Tedec Ulceral;Omid;Zegerid;Result;Ulcesep;Gastroloc;Losec;OmpanytOmebeta;Omizac;Zepral;Demeprazol;Ozoken;Omegast;Zefxon;Danlox;Ceprandal;Olexin;Belmazol;Ulcometion;Erbolin;Miracid;Osiren;Prazolit;Ulcozol;Inhipump;Nilsec;Exter; Density:1.371 g/cm3 Melting Point:156 °C Boiling Point:599.991 °C at 760 mmHg Flash Point:316.663 °C Solubility:Easily soluble in dichloromethane, chloroform and methanol Appearance:white crystalline solid Assay: 99% Package: 25kg/drum Storage: In an airtight container,protected from light, at a temperature between 2℃ and 8℃ Application: ① gastric, duodenal ulcer ②peptic ulcer hemorrhage |
| CAS Registry Number: | 73590-58-6 |
| Synonyms: | ;2-Chloromethyl-3,5-dinmethyl-4-methoxypyridine;5-Methoxy-2-[(4-methoxy-3,5-dimethyl-pyridin-2-yl)methylsulfinyl]-3H-benzoimidazole;ZOLTUM;R-(+)-OMEPRAZOLE;OMEPRAL;MOPRAL;MEPRAL;LOSEC;OMERPRAZOLE; |
| Molecular Formula: | C17H19N3O3S |
| Molecular Weight: | 345.42 |
| Molecular Structure: | 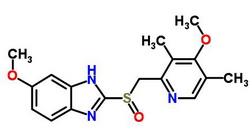
|
| Hazard Symbols: |  Xi:Irritant; Xi:Irritant; |
| Risk Codes: | R36/37/38:; |
| Safety Description: | S26:; S36:; |
| Company: | Wuhan Yuancheng Technology Development Co., Ltd. [ China ] |
| Contact: | Amy |
| Tel: | 18872220679 |
| Fax: | |
| Email: | summer@ycphar.com> |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


