Home > Offer to Sell > Intermediates > Pharmaceutical intermediates > Phenolphthalein CAS 77-09-8 for Anthelmintic (jerryzhang001@chembj.com)
Phenolphthalein CAS 77-09-8 for Anthelmintic (jerryzhang001@chembj.com)
Inquiry
| Post Date: | Mar 24,2017 |
| Expiry Date: | Mar 24,2018 |
| Detailed Description: |
Cas No. :77-09-8
Phenolphthalein CAS 77-09-8 for Anthelmintic
Product Name: Phenolphthalein; Synonyms: LABOTEST-BB LT02090809;CI 764;CI NO 764 (1924);BETZ 0212;3,3-BIS(4-HYDROXYPHENYL)-1(3H)-ISOBENZOFURANONE;3,3-Bis(4-hydroxyphenyl)phthalide;3,3-BIS(P-HYDROXYPHENYL)PHTHALIDE;TIMTEC-BB SBB008868; CAS: 77-09-8; MF: C20H14O4; MW: 318.32; EINECS: 201-004-7; Product Categories: Furan&Benzofuran;Analytical Chemistry;Indicator (pH);pH Indicators;Chemistry;Indicator Solutions;Indicators;Titration;Indicator SolutionsStains and Dyes;P;Stains&Dyes, A to;API; Melting point: 258-263 °C; density: 1.299; Fp: 24 °C; Water Solubility: <0.1 g/100 mL; HS Code: 29322910; Chemical Properties: white to light yellow crystal powder; Description: Phenolphthalein is a chemical compound with the formula C20H14O4 and is often written as "HIn" or "phph" in shorthand notation. Phenolphthalein is often used as an indicator in acid-base titrations. For this application, it turns colorless in acidic solutions and pink in basic solutions. Phenolphthalein is slightly soluble in water and usually is dissolved in alcohols for use in experiments. It is a weak acid, which can lose H+ ions in solution. The phenolphthalein molecule is colorless, and the phenolphthalein ion is pink. When a base is added to the phenolphthalein, the molecule ? ions equilibrium shifts to the right, leading to more ionization as H+ ions are removed. This is predicted by Le Chatelier's principle. Usage: Phenolphthalein's common use is as an indicator in acid-base titrations. It also serves as a component of universal indicator, together with methyl red, bromothymol blue, and thymol blue. Phenolphthalein adopts four different states in aqueous solution: Under very strongly acidic conditions, it exists in protonated form, providing an orange coloration. Between strongly acidic and slightly basic conditions, the lactone form is colorless. The singly deprotonated phenolate form (the anion form of phenol) gives the familiar pink color. In strongly basic solutions, phenolphthalein's pink color undergoes a rather slow fading reaction and becomes completely colorless above 13.0 pH. The rather slow fading reaction that produces the colorless InOH3? ion is sometimes used in classes for the study of reaction kinetics. |
| CAS Registry Number: | 77-09-8 |
| Synonyms: | ;Phenolphthalein solution;3,3-bis(p-hydroxyphenyl)phthalide;,3-bis(4-hydroxyphenyl)-1(3H)-isobenzofuranore;3,3-bis(4-hydroxyphenyl)-2-benzofuran-1(3H)-one;2-[bis(4-hydroxyphenyl)methyl]benzoic acid;Phenolphthalien; |
| Molecular Formula: | C20H14O4 |
| Molecular Weight: | 318.3287 |
| Molecular Structure: | 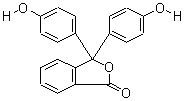
|
| Hazard Symbols: |  Xn:Harmful; Xn:Harmful; |
| Risk Codes: | R22:; R40:; |
| Safety Description: | S36/37:; S45:; |
| Company: | Nanjing Bangnuo Biotechnology Co., Ltd. [ China ] |
| Contact: | Jerry Zhang |
| Tel: | 86-25-52198306 |
| Fax: | 86-25-52198306 |
| Email: | jerryzhang001@chembj.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


