Pioglitazone hydrochloride
Inquiry
| Post Date: | Jan 09,2018 |
| Expiry Date: | Jul 08,2018 |
| Detailed Description: |
Cas No. :112529-15-4
Quantity: mass in stockKilograms Specs:white powder Price:negotiable USD Kilograms Payment Method: T/T, Western Union,Moneygram and Bitcoin. Pioglitazone (brand name Actos) is a prescription drug of the thiazolidinedione (TZD) class with hypoglycemic (antihyperglycemic, antidiabetic) action to treat diabetes. While pioglitazone does decrease blood sugar levels, studies on the main cardiovascular outcomes have not yielded statistically significant results. Its cardiovascular safety profile compares favorably with that of rosiglitazone, which was withdrawn from some markets after concerns about an increased risk of cardiac events. Pioglitazone has been found to be associated with bladder tumors. It has been withdrawn in some countries. Pioglitazone is used to lower blood glucose levels in the treatment of diabetes mellitus type 2 (T2DM) either alone or in combination with a sulfonylurea, metformin, or insulin. The main study that looked at the medication, however, found no statistically significant difference in the main cardiovascular outcomes that were looked at. The secondary outcome of death from all causes, myocardial infarction, and stroke were lower. Pioglitazone has also been used to treat non-alcoholic steatohepatitis (fatty liver), but this use is presently considered experimental. Pioglitazone cannot be used in patients with a known hypersensitivity to pioglitazone, other thiazolidinediones or any of components of its pharmaceutical forms. It is ineffective and possibly harmful in diabetes mellitus type 1 and diabetic ketoacidosis. Its safety in pregnancy, lactation (breastfeeding) and people under 18 is not established. Given previous experiences with the related drug troglitazone, acute diseases of the liver are regarded as a contraindication for pioglitazone. Pioglitazone and all other drugs of its class (thiazolidinediones) are absolutely contraindicated in patients with heart failure. A press release by GlaxoSmithKline in February 2007 noted that there is a greater incidence of fractures of the upper arms, hands and feet in female diabetics given rosiglitazone compared with those given metformin or glyburide. The information was based on data from the ADOPT trial. Following release of this statement, Takeda Pharmaceutical Company, the developer of pioglitazone (sold as Actos in many markets) admitted that it has similar implications for female patients. The risk of hypoglycemia is low in the absence of other drugs that lower blood glucose. Pioglitazone can cause fluid retention and peripheral edema. As a result, it may precipitate congestive heart failure (which worsens with fluid overload in those at risk). It may cause anemia. Mild weight gain is common due to increase in subcutaneous adipose tissue. In studies, patients on pioglitazone had an increased proportion of upper respiratory tract infection, sinusitis, headache, myalgia and tooth problems. Chronic administration of the drug has led to occasional instances of cholestatic hepatitis, reversible upon drug discontinuation. On July 30, 2007 an Advisory Committee of the Food and Drug Administration concluded that the use of rosiglitazone for the treatment of type 2 diabetes was associated with a greater risk of "myocardial ischemic events" when compared to placebo, but when compared to other diabetes drugs, there was no increased risk. Pioglitazone is currently being reviewed. A meta-analysis released subsequently showed that pioglitazone reduced the risk of ischemic cardiac events rather than increased the risk, but increased CHF. On June 9, 2011 the French Agency for the Safety of Health Products decided to withdraw pioglitazone in regards to high risk of bladder cancer. This suspension was based on the results of an epidemiological study conducted by the French National Health Insurance. According to the results of the epidemiological study, the French agency found that patients, who were taking Actos for a long time to aid in type 2 diabetes mellitus, significantly increased risk of bladder cancer compared with patients who were taking other diabetes medications. On June 10, 2011 Germany's Federal Institute for Drugs and Medical Devices also advised doctors not to prescribe the medication until further investigation of the cancer risk had been conducted. On June 15, 2011 the U.S. FDA announced that pioglitazone use for more than one year may be associated with an increased risk of bladder cancer, and two months later the label was updated with an additional warning about this risk. |
| CAS Registry Number: | 112529-15-4 |
| Synonyms: | ;Pioglitazone HCl;5-[[4-[2-(5-Ethyl-2-pyridinyl]ethoxy]phenyl]methyl]-2,4-thiazolidinedione N-Oxide;Pioglitazone N-Oxide;5-{4-[2-(5-ethyl-1-oxidopyridin-2-yl)ethoxy]benzyl}-1,3-thiazolidine-2,4-dione;5-[[4-2-(5-ethyl-2-pyridinyl)ethoxy]benzylidene]-2,4-thiazolidinedione Hydrochloride;[5-[[4-[2-(5-Ethyl-2-Pyridinyl)Ethoxy]Phenyl]Methyl]-2,4-] Thiazolidinedione Hydrochloride; |
| Molecular Formula: | C19H20N2O3S·HCl |
| Molecular Weight: | 392.90 |
| Molecular Structure: | 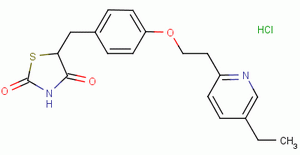
|
| Company: | Hengyang Desen Biotechnology Co., Ltd. [ China ] |
| Contact: | ava |
| Tel: | 86-18908446905 |
| Fax: | |
| Email: | ava@ycgmp.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.