Pitavastatin Calcium
Inquiry
| Post Date: | May 08,2025 | |||||||||||||||||||||||||||||||||||||||||
| Expiry Date: | Nov 04,2025 | |||||||||||||||||||||||||||||||||||||||||
| Detailed Description: |
Cas No. :147526-32-7
Assay: ≥98.5% Cas: 147526-32-7 Usage: Use to made Antihyperlipidemia
|
|||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number: | 147526-32-7 |
| Synonyms: | ;(+)-Monocalciumbis{(3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-3,5-dihydroxy-6-heptenoate};calcium bis{(1R,3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-1,3,5-trihydroxyhept-6-en-1-olate};(+)-Monocalciumbis{(3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-,5-dihydroxy-6-heptenoate}; |
| Molecular Formula: | C50H48CaF2N2O8 |
| Molecular Weight: | 882.9995264 |
| Molecular Structure: | 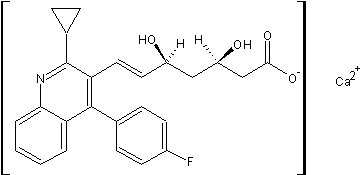
|
| Company: | Quzhou Aifeimu Chemical Co., Ltd. [ China ] |
| Contact: | David chen |
| Tel: | +86-570-6040288 |
| Fax: | +86-570-6040488 |
| Email: | sales@afmpharm.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.

-
- C-2
- C-1
- (E)-3-[3-(4-Fluorophenyl)-...
- (4R-cis)-1,1-Dimethylethyl...
- Bis[(3R,5S,6E)-7-(2-Cyclop...
- (4R-Cis)-6-Hydroxymethyl-2...
- 4-(4-Fluorophenyl)-6-Isopr...
- (R)-4-Cyano-3-Hydroxybutyr...
- R-1-3
- Tert-butyl-(+)7-[4-(4-fluo...
- R-3
- N-1
- Ethyl-2-Cyclopropyl-4-(4-F...
- N-2
- ATS-8
- Pitavastatin Calcium
- R-1-2 Methanol
- F-1
- (4R,Cis)-1,1-Dimethylethy-...
- (3R,5R)-7-[2-(4-Fluorophen...
- 3-(4-Fluorophenyl)-1-(1-Me...
- Tert-butyl(E)-3,5-Dihydrox...
- ATS-5
- Rosuvastatin Calcium
- (4R-Cis)-6-[(Acetyloxy met...

