Home > Offer to Sell > Intermediates > Pharmaceutical intermediates > Safety Dutasteride / Avodart Powder For Women / Men Hair Loss Treatment CAS 164656-23-9
Safety Dutasteride / Avodart Powder For Women / Men Hair Loss Treatment CAS 164656-23-9
Inquiry
| Post Date: | Oct 23,2015 |
| Expiry Date: | Oct 22,2016 |
| Detailed Description: |
Cas No. :164656-23-9
Quantity: 1Kilograms Specs:KOSHER,GMP,SGS,ISO9001 Price:1 USD Kilograms Payment Method: Western Union,Moneygram,Banktransfer,T/T Safety Dutasteride / Avodart Powder For Women / Men Hair Loss Treatment CAS 164656-23-9 Other name:DUTASTERIDE; Duagen; Avodart CAS No: 164656-23-9 MF: C27H30F6N2O2 MW: 528.53 Purity: 99% Appearance: White powder. USES: Medicine material. The treatment of prostate hyperplasia. Description: Dutasteride (trademark name Avodart) is a dual 5-α reductase inhibitor that inhibits conversion of testosterone to dihydrotestosterone (DHT). Used for benign prostatic hyperplasia; however, it increases the risk of erectile dysfunction and decreased sexual desire. Dutasteride useful for the symptoms of benign prostatic hyperplasia (BPH); colloquially known as an "enlarged prostate". In those who are being regularly screened 5-alpha-reductase inhibitor (finasteride and dutasteride) reduce the overall risk of being diagnosed with prostate cancer however there is insufficient data to determine if they have an effect on the risk of death and may increase the chance of more serious cases. This class of medications increases rates of erectile dysfunction (with between 5% and 9% developing problems after starting their use). This is linked to lower quality of life and can cause stress in relationships. There is also an association with lowered sexual desire. It has been reported that these adverse sexual side effects may persist even after discontinuation of the drug. The FDA has added a warning to dutasteride about an increased risk of high-grade prostate cancer. While the potential for positive, negative or neutral changes to the potential risk of developing prostate cancer with dutasteride has not been established, evidence has suggested it may temporarily reduce the growth and prevalence of benign prostate tumors, but could also mask the early detection of prostate cancer. The primary area for concern is for patients who may develop prostate cancer whilst taking dutasteride for benign prostatic hyperplasia, which in turn could delay diagnosis and early treatment of the prostate cancer, thereby potentially increasing the risk of these patients developing high-grade prostate cancer. Email:sunny@chembj.com |
| CAS Registry Number: | 164656-23-9 |
| Synonyms: | ;(5alpha,17beta)-n-{2,5-bis(trifluoromethyl)phenyl}-3-oxo-4-azaandrost-l-ene-17-carboxamide;(4aR,6aS,7S,9aS,9bS,11aR)-N-[2,5-bis(trifluoromethyl)phenyl]-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide;(4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-[2,5-bis(trifluoromethyl)phenyl]-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide;(4aR,6aS,7S)-N-[2,5-bis(trifluoromethyl)phenyl]-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide; |
| Molecular Formula: | C27H30F6N2O2 |
| Molecular Weight: | 528.5297 |
| Molecular Structure: | 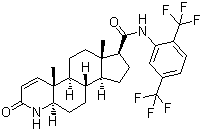
|
| Company: | Hongkong Yuancheng Gongchuang Technology Co.Ltd. [ China ] |
| Contact: | sunny |
| Tel: | 86-027-50756220 |
| Fax: | |
| Email: | sunny@ycphar.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


