Home > Offer to Sell > Intermediates > Pharmaceutical intermediates > Sodium bicarbonate CAS 144-55-8(jerryzhang001@chembj.com)
Sodium bicarbonate CAS 144-55-8(jerryzhang001@chembj.com)
Inquiry
| Post Date: | Apr 20,2017 |
| Expiry Date: | Apr 20,2018 |
| Detailed Description: |
Cas No. :144-55-8
Payment Method: T/T; Western Union; Moneygram and Bitcoin Sodium bicarbonate CAS 144-55-8 Product Name: Sodium bicarbonate; Synonyms: bicarbonatedesodium;col-evac;jusonin;meylon;monosodiumcarbonate;monosodiumhydrogencarbonate;neut;seldevichy; CAS: 144-55-8; MF: CHNaO3; MW: 84.01; EINECS: 205-633-8; Product Categories: INORGANIC & ORGANIC CHEMICALS;FOOD ADDITIVES;Inorganics;Eluent concentrates for IC;Chromatography/CE Reagents;Ion Chromatography;Biological Buffers;Cell Culture;Reagents and Supplements;metal carbonate; Melting point: >300 °C(lit.); Boiling point: 851°C; density: 2.16 g/mL at 25 °C(lit.); refractive index: 1.500; storage temp.: 2-8°C; HS Code: 28363000; Chemical Properties: white powder or crystals; General Description: Odorless white crystalline powder or lumps. Slightly alkaline (bitter) taste. pH (of freshly prepared 0.1 molar aqueous solution): 8.3 at 77°F. pH (of saturated solution): 8-9. Non-toxic. Sodium bicarbonate (IUPAC name: sodium hydrogen carbonate) is a chemical compound with the formula NaHCO3. It is a salt composed of sodium ions and bicarbonate ions. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs. It is among the food additives encoded by the European Union, identified as E 500. Usage: Cooking: Sodium bicarbonate, referred to as baking soda, is primarily used in baking, as a leavening agent. It reacts with acidic components in batters, releasing carbon dioxide, which causes expansion of the batter and forms the characteristic texture and grain in pancakes, cakes, quick breads, soda bread, and other baked and fried foods. Acidic compounds that induce this reaction include phosphates, cream of tartar, lemon juice, yogurt, buttermilk, cocoa and vinegar. Natural acids in sourdough can be leavened with the addition of small amounts as well. Pest control: Used to kill cockroaches. Once consumed, it causes internal organs of cockroaches to burst due to gas collection. Sodium bicarbonate can be an effective way of controlling fungal growth, and in the United States is registered by the Environmental Protection Agency as a biopesticide. Paint and corrosion removal: Sodium bicarbonate is used in a process for removing paint and corrosion called sodablasting; the process is particularly suitable for cleaning aluminium panels which can be distorted by other types of abrasive. Alkalinity/pH increase: It can be administered to pools, spas, and garden ponds to raise the total alkalinity, this will also raise the pH level and make maintaining proper pH easier. In the event that the pH is low and the alkalinity is adequate or high, Baking Soda (sodium bicarbonate) should not be used to adjust the pH. Pyrotechnics: Sodium bicarbonate is one of the main components of the common incendiary "black snake" firework. The effect is caused by the thermal decomposition, which produces carbon dioxide gas to produce a long snake-like ash as a combustion product of the other main component, sucrose. Mild disinfectant: It has weak disinfectant properties,and it may be an effective fungicide against some organisms. Because baking soda will absorb musty smells, it has become a reliable method for used-book sellers when making books less malodorous. Fire extinguisher: Sodium bicarbonate can be used to extinguish small grease or electrical fires by being thrown over the fire, as heating of sodium bicarbonate releases carbon dioxide. However, it should not be applied to fires in deep fryers; the sudden release of gas may cause the grease to splatter.Sodium bicarbonate is used in BC dry chemical fire extinguishers as an alternative to the more corrosive diammonium phosphate in ABC extinguishers. The alkaline nature of sodium bicarbonate makes it the only dry chemical agent, besides Purple-K, that was used in large-scale fire suppression systems installed in commercial kitchens. Because it can act as an alkali, the agent has a mild saponification effect on hot grease, which forms a smothering, soapy foam. |
| CAS Registry Number: | 144-55-8 |
| Synonyms: | ;Baking soda;Bicarbonate of soda;Carbonic acid monosodium salt;col-evac;meylon;monosodium carbonate;monosodium hydrogen carbonate;Sodium acid carbonate;sodium hydrocarbonate;Sodium Hydrogen Carbonate;soda mint;soludal;hydrogen carbonate; |
| Molecular Formula: | NaHCO3 |
| Molecular Weight: | 84.01 |
| Molecular Structure: | 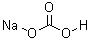
|
| Safety Description: | S24/25:; |
| Company: | Nanjing Bangnuo Biotechnology Co., Ltd. [ China ] |
| Contact: | Jerry Zhang |
| Tel: | 86-25-52198306 |
| Fax: | |
| Email: | jerryzhang001@chembj.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


