Home > Offer to Sell > Pharmaceuticals and Biochemicals > Hormones and synthetic substitutes > Sodium tetraphenylborate,CAS 143-66-8
Sodium tetraphenylborate,CAS 143-66-8
Inquiry
| Post Date: | Nov 25,2016 |
| Expiry Date: | Nov 25,2017 |
| Detailed Description: |
Cas No. :143-66-8
Contact Information:
sophia525 at yccreate dot com Facebook:sophia525 at ycgmp dot com IUPAC name:Sodium tetraphenylborate Other names:Tetraphenylboron sodium Purity:99.4% Packing:20kg/carboard drum Grade:industrial grade Appearance:white powder CAS Registry Number:143-66-8 Chemical formula:(C6H5)4BNa Molar mass:342.216 g/mol Melting point > 300 °C (572 °F; 573 K) Solubility in water:47 g/100 mL Solubility:soluble in ethanol Introduction: Sodium tetraphenylborate is the organic compound with the formula NaB(C6H5)4. It is a salt, wherein the anion consists of four phenyl rings bonded to boron. This white crystalline solid is used to prepare other tetraphenylborate salts, which are often highly soluble in organic solvents. The compound is used in inorganic and organometallic chemistry as a precipitating agent. Use in chemical synthesis Preparation of N-acylammonium salts Addition of sodium tetraphenylborate to a solution of a tertiary amine and an acid chloride in acetonitrile gives the acylonium salt by precipitating NaCl from the reaction mixture. This method has a broad scope: RC(O)Cl R'3N NaB(C6H5)4 → [RC(O)NR'3][B(C6H5)4] NaCl Sodium tetraphenylborate is also employed as a phenyl donor in palladium-catalyzed cross-coupling reactions involving vinyl and aryl triflates to give arylalkenes and biaryl compounds in good yields and under mild conditions, respectively. Use in coordination chemistry Tetraphenylborates are often studied in organometallic chemistry because of their favorable solubility in nonpolar solvents and their crystallinity. For example, the homoleptic trimethylphosphite complexes {M[P(OCH3)3]5}2 (Ni, Pd, and Pt) have been prepared as their tetraphenylborate salts.Similarly, sodium tetraphenylborate has been used to isolate complexes containing dinitrogen ligands. In the reaction below, sodium tetraphenylborate allows N2 to displace the chloride ligand, which is removed from solution as a precipitate of sodium chloride: FeHCl(diphosphine)2 NaB(C6H5)4 N2 → [FeH(N2)(diphosphine)2]B(C6H5)4 NaCl The use of tetraphenylborate is limited to non-acidic cations. With strong acids, the anion undergoes protonolysis to give triphenylborane and benzene:[8] H B(C6H5)4- → B(C6H5)3 C6H6 Packaging & Delivery: 1.Sufficient stock. We can delivery promptly at the very day when receive the payment 2.Sophisticated and professional logistic agent. We take responsibility to provide our customers with fast delivery and secure shipping 3.Well-trained and disciplined packing team. Unique ways to ship 10 grams to 100kg powders at one time to your destination. Fast and discreet shipment could be arranged for customs pass Guaranteed. 4.Packing pictures and tacking code are provided within 12 hours after receiving the payment. Updated tracking information will be provided every other day. 5.After-sale service: Any questions or problems after receiving the product, please feel free to contact us. Problems would be solved immediately. |
| CAS Registry Number: | 143-66-8 |
| Synonyms: | ;Sodium tetraphenylborate;Sodium tetraphenylboron;Borate(1-),tetraphenyl-,sodium;kariporuk;sodiumtetraphenylborate(1-);sodiumtetraphenylborate(naph4b);sodiumtetraphenylboride;sodiumtetraphenylboride(1-);tetraphenyl-borate(1-sodium;tetraphenyl-borate(1-sodiumsalt;sodium tetraphenylborate(1-);tetraphenylborate(1-);cyclohex-1-yliumyl(triphenyl)borate(1-); |
| Molecular Formula: | C24H20BNa |
| Molecular Weight: | 342.22 |
| Molecular Structure: | 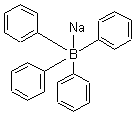
|
| Hazard Symbols: |  Xn:Harmful; Xn:Harmful; |
| Risk Codes: | R22:; |
| Company: | Wuhan Yuancheng Gongchuang Technology Co., Ltd. [ China ] |
| Contact: | Sophia Zhang |
| Tel: | +86-027-50756082 |
| Fax: | |
| Email: | sophia525@yccreate.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


