Sorafenib tosylate
Inquiry
| Post Date: | Jan 09,2018 |
| Expiry Date: | Jul 08,2018 |
| Detailed Description: |
Cas No. :475207-59-1
Quantity: mass in stockKilograms Specs:white powder Price:negotiable USD Kilograms Payment Method: T/T, Western Union,Moneygram and Bitcoin. Quick Details: Product Name: Sorafenib tosylate CAS NO: 475207-59-1 Appearance: white powder MF: C21H16ClF3N4O3.C7H8SO3 Purity: 99% Origin: China Export market: Global Grade: Pharmaceutical grade Supply capacity: Mass in stock. Usage: Anti-cancer drugs Packing: Well disguised package to pass the customs. Payment: T/T, Western Union,Moneygram and Bitcoin. Shiping: EMS,HKEMS,FEDEX,DHL,TNT,Aramex,etc Leading time: Within 24 hours after payment. Delivery time: 3-7 work days 99% passing rate & 100% free resend policy, Description: Sorafenib (co-developed and co-marketed by Bayer and Onyx Pharmaceuticals as Nexavar), is a kinase inhibitor drug approved for the treatment of primary kidney cancer (advanced renal cell carcinoma), advanced primary liver cancer (hepatocellular carcinoma), and radioactive iodine resistant advanced thyroid carcinoma. Mechanism of action Sorafenib is a small inhibitor of several tyrosine protein kinases, such as VEGFR, PDGFR and Raf family kinases (more avidly C-Raf than B-Raf). Sorafenib treatment induces autophagy, which may suppress tumor growth. However, autophagy can also cause drug resistance. Medical uses At the current time sorafenib is indicated as a treatment for advanced renal cell carcinoma (RCC), unresectable hepatocellular carcinomas (HCC) and thyroid cancer. Kidney cancer Clinical trial results, published January 2007, showed that, compared with placebo, treatment with sorafenib prolongs progression-free survival in patients with advanced clear cell renal cell carcinoma in whom previous therapy has failed. The median progression-free survival was 5.5 months in the sorafenib group and 2.8 months in the placebo group. In Australia this is one of two TGA-labelled indications for sorafenib, although it is not listed on the Pharmaceutical Benefits Scheme for this indication. Liver cancer At ASCO 2007, results from the SHARP trial were presented, which showed efficacy of sorafenib in hepatocellular carcinoma. The primary endpoint was median overall survival, which showed a 44% improvement in patients who received sorafenib compared to placebo (hazard ratio 0.69; 95% CI, 0.55 to 0.87; p=0.0001). Both median survival and time to progression showed 3-month improvements; however, there was no significant difference in median time to symptomatic progression (p=0.77). There was no difference in quality of life measures, possibly attributable to toxicity of sorafenib or symptoms related to underlying progression of liver disease. Of note, this trial only included patients with Child-Pugh Class A (i.e. mildest) cirrhosis.Because of this trial Sorafenib obtained FDA approval for the treatment of advanced hepatocellular carcinoma in November 2007. In a randomized, double-blind, phase II trial combining sorafenib with doxorubicin, the median time to progression was not significantly delayed compared with doxorubicin alone in patients with advanced hepatocellular carcinoma. Median durations of overall survival and progression-free survival were significantly longer in patients receiving sorafenib plus doxorubicin than in those receiving doxorubicin alone. A prospective single-centre phase II study which included the patients with unresectable hepatocellular carcinoma (HCC)concluding that the combination of sorafenib and DEB-TACE in patients with unresectable HCC is well tolerated and safe, with most toxicities related to sorafenib. In Australia this is the only indication for which sorafenib is listed on the PBS and hence the only Government-subsidised indication for sorafenib. Along with renal cell carcinoma, hepatocellular carcinoma is one of the TGA-labelled indications for sorafenib. Thyroid cancer On November 22, 2013, sorafenib was approved by the FDA for the treatment of locally recurrent or metastatic, progressive differentiated thyroid carcinoma (DTC) refractory to radioactive iodine treatment. The Phase 3 DECISION trial showed significant improvement in progression-free survival but not in overall survival. However, as is known, the side effects were very frequent, specially hand and foot skin reaction. Desmoid tumors A phase 3 clinical trial is under way testing the effectiveness of Sorafenib to treat desmoid tumors (also known as aggressive fibromatosis), after positive results in the first two trial stages. Dosage is typically half of that applied for malignant cancers (400 mg vs 800 mg). NCI are sponsoring this trial. |
| CAS Registry Number: | 475207-59-1 |
| Synonyms: | ;SORAFENIB-D3;4-Methyl-3-((4-(3-pyridinyl)-2-pyrimidinyl)amino)-N-(5-(4-methyl-1H-imidazol-1-yl)-3-(trifluoromethyl)phenyl)benzamide monomethanesulfonate;N-[4-Chloro-3-(trifluoromethyl)phenyl]-N'-[4-[2-(N-methylcarbamoyl)-4-pyridyloxy]phenyl]urea Tolsylate;Sorafenib TsOH salt; |
| Molecular Formula: | C21H16ClF3N4O3·C7H8SO |
| Molecular Weight: | 637.03 |
| Molecular Structure: | 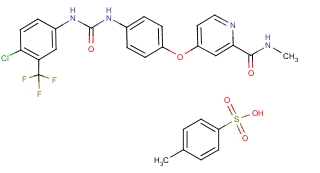
|
| Company: | Hengyang Desen Biotechnology Co., Ltd. [ China ] |
| Contact: | ava |
| Tel: | 86-18908446905 |
| Fax: | |
| Email: | ava@ycgmp.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


