Tetracaine
Inquiry
| Post Date: | Nov 18,2017 |
| Expiry Date: | May 17,2018 |
| Detailed Description: |
Cas No. :94-24-6
Quantity: mass in stockKilograms Specs:white powder Price:negotiable USD Kilograms Payment Method: T/T, Western Union,Moneygram and Bitcoin. Description: Tetracaine (INN, also known as amethocaine; trade name Pontocaine. Ametop and Dicaine) is a potent local anesthetic of the ester group. It is mainly used topically in ophthalmology and as an antipruritic, and it has been used in spinal anesthesia. It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system. 1) Tetracaine is a local anesthetic (numbing medicine). It works by blocking nerve signals in your body. 2) Tetracaine topical may also be used for purposes not listed in this medication guide. 3) Tetracaine topical (for the skin) is used to numb different parts of the body before a medical test or procedure. 4) In the manufacture of pharmaceutical preparation for external use, to relieve the pain of drugs or medication lesions pain. Applications: (1) In biomedical research, tetracaine is used to alter the function of calcium release channels (ryanodine receptors) that control the release of calcium from intracellular stores. Tetracaine is an allosteric blocker of channel function. At low concentrations, tetracaine causes an initial inhibition of spontaneous calcium release events, while at high concentrations, tetracaine blocks release completely. (2) Tetracaine is the T in Tac, a mixture of 5 to 12 per cent tetracaine, 5M(per myriad), a half per mille (0.5‰), or .05 per cent (1 part in 2000) , and 4 or 10 per cent hydrochloride used in ear, nose & throat surgery and in the emergemcy department where numbing of the surface is needed rapidly, especially when children have been injured in the eye, ear, or other sensitive locations. (3) Tetracaine is synthesized from 4-butylaminobenzoic acid. The ethyl ester is formed through an acid-catalyzed esterification reaction. Base-catalyzed transesterification is achieved by boiling the ethyl ester of 4-butylaminobenzoic acid with excess 2-dimethylaminoethanol in the presence of a small amount of sodium ethoxide. |
| CAS Registry Number: | 94-24-6 |
| Synonyms: | ;tetracaine usp;tetracaine free base;Tetracaine Base;amethocaine;Dimethylaminoethylnbutylaminobenzoate;Tetracaine;2-(dimethylamino)ethyl 4-(butylamino)benzoate; |
| Molecular Formula: | C15H24N2O2 |
| Molecular Weight: | 264.3633 |
| Molecular Structure: | 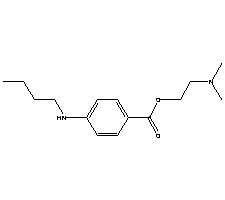
|
| Hazard Symbols: |  Xn:Harmful; Xn:Harmful; |
| Risk Codes: | R22:; R40:; R42/43:; |
| Safety Description: | S22:; S36/37:; |
| Company: | Hengyang Desen Science and Technology co., LTD. [ China ] |
| Contact: | Eric yang |
| Tel: | +86-189-08446905 |
| Fax: | |
| Email: | Eric@desen-nutrition.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


