Home > Offer to Sell > Pharmaceuticals and Biochemicals > Antibiotic and antimicrobial agents > Top Quality Anesthesia Medicine Lidocaine Factory Supplying
Top Quality Anesthesia Medicine Lidocaine Factory Supplying
Inquiry
| Post Date: | Jun 09,2015 |
| Expiry Date: | Jun 08,2016 |
| Detailed Description: |
Cas No. :137-58-6
Quantity: 1000Metric Tons Specs:1kg/ Foil bay or as custome's requirement Price:1 USD Kilograms Payment Method: T/T, Western Union, Money Gram Product Name: Lidocaine Tag: Lidocaine, Xylocaine 1. Basic Information: Product Name Lidocaine Synonym Xylocaine,2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide CAS 137-58-6 MF C14H22N2O MW 234.34 EINECS 205-302-8 Assay 99% Quality Standards Enterprise Standard/Pharma Grade Appearance White powder Package 25kg/Drum Place of Origin China Brand Name SMQ Certification ISO9001, ISO14000, KOSHER Model Number 137-58-6 MOQ 1kg Ref. Price USD 38/kg Packaging Details 25kg/Drum, 1kg/bag Delivery Time 1-2 days Payment Terms T/T, Western union, Money gram Supply Ability 500000 T/Month 2. Quick View: Lidocaine (INN, BAN) xylocaine, or lignocaine (AAN, former BAN) is a common local anesthetic and class-1b antiarrhythmic drug. Lidocaine is used topically to relieve itching, burning, and pain from skin inflammations, injected as a dental anesthetic, or as a local anesthetic for minor surgery. It is on the World Health Organization's List of Essential Medicines, a list of the most important medications needed in a basic healthcare system. 3. History: Lidocaine, the first amino amide–type local anesthetic, was first synthesized under the name 'xylocaine' by Swedish chemist Nils L?fgren in 1943. His colleague Bengt Lundqvist performed the first injection anesthesia experiments on himself. It was first marketed in 1949. 4. Pharmacodynamics: (1) Anaesthesia Lidocaine alters signal conduction in neurons by blocking the fast voltage-gated Na+ channels in the neuronal cell membrane responsible for signal propagation. With sufficient blockage, the membrane of the postsynaptic neuron will not depolarize and will thus fail to transmit an action potential. This creates the anaesthetic effect by not merely preventing pain signals from propagating to the brain, but by stopping them before they begin. Careful titration allows for a high degree of selectivity in the blockage of sensory neurons, whereas higher concentrations also affect other modalities of neuron signaling. (2) Antiarrhythmic The same principle applies for this drug's actions in the heart. Blocking sodium channels in the conduction system, as well as the muscle cells of the heart, raises the depolarization threshold, making the heart less likely to initiate or conduct early action potentials that may cause an arrhythmia. 5. Pharmacokinetics: The onset of action of lidocaine is about 45 to 90 sec and its duration is 10 to 20 min. It is about 95% metabolized (dealkylated) in the liver mainly by CYP3A4 to the pharmacologically active metabolites monoethylglycinexylidide (MEGX) and then subsequently to the inactive glycine xylidide. MEGX has a longer half-life than lidocaine, but also is a less potent sodium channel blocker. The volume of distribution is 1.1-2.1 l/kg, but congestive heart failure can decrease it. About 60-80% circulates bound to the protein alpha1 acid glycoprotein. The oral bioavailability is 35% and the topical bioavailability is 3%. The elimination half-life of lidocaine is biphasic and around 90–120 min in most patients. This may be prolonged in patients with hepatic impairment (average 343 min) or congestive heart failure (average 136 min). Lidocaine is excreted in the urine (90% as metabolites and 10% as unchanged drug). 6. Medical uses: (1) The efficacy profile of lidocaine as a local anesthetic is characterized by a rapid onset of action and intermediate duration of efficacy. Therefore, lidocaine is suitable for infiltration, block, and surface anesthesia. Longer-acting substances such as bupivacaine are sometimes given preference for subdural and epidural anesthesias; lidocaine, though, has the advantage of a rapid onset of action. Epinephrine (adrenaline) vasoconstricts arteries, reducing bleeding and also delays the resorption of lidocaine, almost doubling the duration of anaesthesia. For surface anesthesia, several available formulations can be used e.g. for endoscopies, before intubations, etc. Buffering the pH of lidocaine makes local freezing less painful. Lidocaine drops can be used on the eyes for short ophthalmic procedures. (2) Topical lidocaine has been shown in some patients to relieve the pain of postherpetic neuralgia (a complication of shingles), though not enough study evidence exists to recommend it as a first-line treatment.[3] Intravenous lidocaine also has uses as a temporary fix for tinnitus. Although not completely curing the disorder, it has been shown to reduce the effects by around two-thirds. (3) Lidocaine is also the most important class-1b antiarrhythmic drug; it is used intravenously for the treatment of ventricular arrhythmias (for acute myocardial infarction, digoxin poisoning, cardioversion, or cardiac catheterization) if amiodarone is not available or contraindicated. Lidocaine should be given for this indication after defibrillation, CPR, and vasopressors have been initiated. A routine prophylactic administration is no longer recommended for acute cardiac infarction; the overall benefit of this measure is not convincing. (4) Inhaled lidocaine can be used as an antitussive (cough suppressor) acting peripherally to reduce the cough reflex. This application can be implemented as a safety and comfort measure for patients who have to be intubated, as it reduces the incidence of coughing and any tracheal damage it might cause when emerging from anesthesia. (5) Lidocaine, along with ethanol, ammonia, and acetic acid, has also been proven to be effective in treating jellyfish stings, both numbing the affected area and preventing further nematocyst discharge. |
| CAS Registry Number: | 137-58-6 |
| Synonyms: | ;2-(Diethylamino)-N-(2,6-dimethylphenyl)-acetamide;Lignocaine;2-diethylaminoacet-2,6-xylidide;Xylocaine;N-(2,6-dimethylphenyl)-N~2~,N~2~-diethylglycinamide;2-[(2,6-dimethylphenyl)amino]-N,N-diethyl-2-oxoethanaminium;Lidocaine base; |
| Molecular Formula: | C14H23N2O |
| Molecular Weight: | 235.3447 |
| Molecular Structure: | 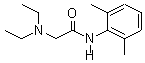
|
| Hazard Symbols: |  Xn:Harmful; Xn:Harmful; |
| Risk Codes: | R22:; |
| Safety Description: | S22:; S26:; S36:; |
| Company: | Hubei Yuchen Pharma Co,. Ltd [ China ] |
| Contact: | dingjianhui |
| Tel: | 86-027-50756254 |
| Fax: | 86-027-68886696 |
| Email: | anastasia@chembj.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


