Home > Offer to Sell > Pharmaceuticals and Biochemicals > Hormones and synthetic substitutes > Vapreotide Acetate
Vapreotide Acetate
Inquiry
| Post Date: | Oct 09,2016 |
| Expiry Date: | Apr 07,2017 |
| Detailed Description: |
Cas No. :103222-11-3
Quantity: 1Kilograms Price:1 USD Kilograms Payment Method: T/T Product Name Vapreotide Acetate Sequence D-Phe-[Cys-Tyr-D-Trp-Lys-Val-Cys]-Trp-NH2 Cas No. 103222-11-3 Molecular Formula C57H70N12O9S2 Molecular Weight 1131.40 Purity (HPLC) 98.0% Appearance? White powder Single Impurity(HPLC)? 1.0% Amino Acid Composition? 10% of theoretical Peptide Content(N%) 80%(by %N) Water Content(Karl Fischer)? 6.0% Acetate Content(HPIC) 15.0% MS(ESI) Mass Balance? 95.0~105.0% 2. Description: Variceal bleeding is a life-threatening complication of portal hypertension. The recommended treatment includes the early administration of a vasoactive drug. Vapreotide is a somatostatin analogue with a different receptor affinity to octreotide. It decreases portal pressure and blood flow of collateral circulation in rats with cirrhosis. The pivotal study of early administration of vapreotide in patients with cirrhosis and variceal bleeding has shown a significant improvement in bleeding control and, in the subset of patients with significant bleeding, a significant reduction in mortality. In addition, a meta-analysis of four randomized studies has shown a significant improvement in bleeding control. Vapreotide administrated via the intravenous route is simple to use, with practically no contraindications and few, usually minor, side effects. 3. Application: The immediate release formulation of Sanvar, a somatostatin analogue, is used in the treatment of acute esophageal variceal bleeding (EVB). Sanvar is used prior to endoscopic intervention to control haemorrhage and prevent re-bleeding during the critical five days following the onset of bleeding. EVB is a life threatening condition and the mortality rate is high (about 15% to 25%) in the first six weeks following the haemorrhage. EVB is the cause of about 70% of gastro-intestinal bleeding in patients suffering from liver cirrhosis. Sanvar (vapreotide acetate) is a synthetic octapeptide analogue of the naturally-occurring somatostatin hormone. It has similar pharmacological properties to native somatostatin, but exhibits a longer duration of action. It is the only somatostatin analogue to have demonstrated statistically significant benefits in the early treatment of EVB in association with endoscopic therapy, in a placebo-controlled clinical study (Calè S et al. New England Journal of Medicine, 2001). Control of bleeding with survival at five days was achieved more often with Sanvar (p=0.021) than with placebo. Additional Phase III trials have been completed in Europe in this indication. Sanvar can be stored at room temperature, an advantage over other products requiring refrigeration, allowing immediate administration, a key benefit in a life-threatening situation. The product has been granted orphan drug status in the U. S., where there is currently no FDA approved treatment for this indication. |
| CAS Registry Number: | 103222-11-3 |
| Synonyms: | Vapreotide [USAN:INN];BMY 41606;CCRIS 6495;D-Phenylalanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-L-tryptophanamide cyclic (2-7)-disulfide;D-Phenylalanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-L-tryptophanamide cyclic (2->7)-disulfide;L-Tryptophanamide, D-phenylalanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-, cyclic (2-7)-disulfide;RC 160;Sanvar IR;UNII-2PK59M9GFF;Vapreotida;Vapreotida [INN-Spanish];Vapreotidum;Vapreotidum [INN-Latin];L-Tryptophanamide, D-phenylalanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-, cyclic (2->7)-disulfide;10-(4-aminobutyl)-N-[1-amino-3-(1H-indol-3-yl)-1-oxopropan-2-yl]-16-(4-hydroxybenzyl)-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-19-(phenylalanylamino)-7-(propan-2-yl)-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carboxamide; |
| Molecular Formula: | C57H70N12O9S2 |
| Molecular Weight: | 1131.3707 |
| Molecular Structure: | 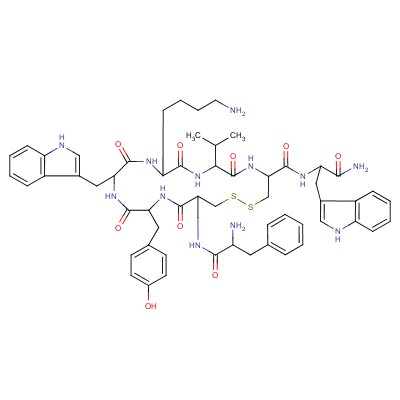
|
| Company: | Hubei Yuancheng Saichuang Technology Co.,Ltd. [ China ] |
| Contact: | fion |
| Tel: | +86-027-50755967 |
| Fax: | |
| Email: | daisy@ycphar.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


