Home > Offer to Sell > Pharmaceuticals and Biochemicals > Hormones and synthetic substitutes > Weight loss diet ingredient Rimonabant
Weight loss diet ingredient Rimonabant
Inquiry
| Post Date: | Dec 07,2016 |
| Expiry Date: | Dec 14,2016 |
| Detailed Description: |
Cas No. :168273-06-1
Quantity: 100Kilograms Payment Method: Western union, money gram, T/T Use: Rimonabant is a weight loss drug, can significantly reduce weight, reduce waist circumference and reduce risk factors for cardiovascular disease, but also can improve blood lipids and insulin resistance and metabolic syndrome, especially for the presence of clinical obesity or heart disease people at risk. Rimonabant as a new weight loss drug, can also be used to quit smoking. Not only lose weight, but also lower blood pressure, cholesterol and blood sugar, clinical study found that subjects taking one to two months later, the weight can be reduced on average six to eight kilograms. |
| CAS Registry Number: | 168273-06-1 |
| Synonyms: | ;ACOMPLIA;RIMONABANT(ACOMPLIA,SR141716);5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidinopyrazole-3-carboxamide;5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide hydrochloride (1:1);5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide; |
| Molecular Formula: | C22H21Cl3N4O |
| Molecular Weight: | 463.7873 |
| Molecular Structure: | 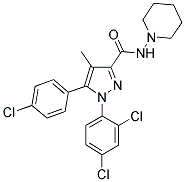
|
| Company: | HUBEI YUANCHENG SAICHUANG TECHNOLOGY CO., LTD [ China ] |
| Contact: | Catherine Lee |
| Tel: | +86-18872220686 |
| Fax: | 86-027-68886696 |
| Email: | 2355916854@ycphar.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.


