Ammonium Bicarbonate
-
Post Date:
May 22,2016 -
Expiry Date:
Nov 18,2016 -
Detailed Description:
Cas No. :1066-33-7Definition:Ammonium bicarbonate is formed as shown below, or by passing carbon dioxide through a solution of the normal compound, when it is deposited as a white powder, which has no smell and is only slightly soluble in water. The aqueous solution of this salt liberates carbon dioxide on exposure to air or on heating, and becomes alkaline in reaction. The aqueous solutions of all the carbonates when boiled undergo decomposition with liberation of carbon dioxide and the substance with which the carbonate ion reacted to form the bicarbonate, in this case, ammonia: NH4HCO3 → NH3 + H2O + CO2
Packing & Loading:25KG PP WOVEN BAGS / 24MTITEMSPECIFICATIONNH4HCO3 %99.2%-101%CL≤0.003%SO4≤0.007%ASH≤0.008%AS≤0.0002%PB≤0.0005% -
CAS Registry Number:
1066-33-7 -
Synonyms:
;Ammonium hydrogen carbonate;acid ammonium carbonate;Ammonium acid carbonate;Carbonic Acid, Monoammonium Salt;ammonium zinc carbonate (2:2:3);Ammoninum Bicarbonate;Ammonium hydrogenocarbonate; -
Molecular Formula:
NH4HCO3 -
Molecular Weight:
79.05 -
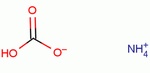
Molecular Structure:
-
Hazard Symbols:
Xi:Irritant;
-
Risk Codes:
R36/37/38:;
-
Safety Description:
S22:不要吸入粉尘;
-
Company:
Zibo Saibo Import & Export Co., Ltd [ China ] -
Contact:
Ms Chen -
Tel:
0533-5201430 -
Fax:
0533-5202046 -
Email:
manager@sinosaibo.com